Volume 12 - Year 2025 - Pages 28-35
DOI: 10.11159/jbeb.2025.004
Neurotoxic Effects of Artificial Food Dye Yellow 5 on Mouse Hippocampal and Cortical Neurons
Ryan Liu¹†,Sarah Chen¹†, Henry Hou¹†, Chengbiao Wu², Linda Shi¹,*, Veronica Gomez-Godinez¹
1Institute of Engineering in Medicine, University of California, San Diego, La Jolla, CA 92093
2Department of Neurosciences, University of California, San Diego, La Jolla, CA 92093
*Correspondence: zshi@ucsd.edu
†Participated in the UCSD IEM OPALS internship
Abstract - The widespread use of artificial food dyes has been recognized as a significant health risk in complicated neurological issues, particularly in children. In this study, we investigated the effects of Yellow 5 (Tartrazine) on cultured mouse cortical and hippocampal neurons. We combined Fluo-4 calcium imaging and laser-induced shockwave injury to examine possible dye-related alterations in intracellular calcium signalling in neurons. Our results demonstrate that hippocampal neurons that were treated with Yellow 5 and Methyl Yellow showed an increase in calcium signals, suggesting these yellow food dyes increase these neurons’ vulnerability to mechanical stress. Treating cortical neurons with these dyes altered calcium kinetics, with some variability depending on the solvent condition. Taken together, our findings provide evidence that food dyes might influence neuronal activity and injury response. We conclude that artificial food dye exposure can potentially induce neuronal damage, highlighting the need for further studies to define the impacts of the artificial food dye on brain development and function.
Keywords: Neurotoxicity, artificial food dye, calcium imaging, hippocampal neurons, cortical neurons, shockwave injury.
© Copyright 2025 Authors This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2025-01-24
Date Revised: 2025-09-11
Date Accepted: 2025-09-24
Date Published: 2025-10-30
1. Introduction
Artificial food dyes have been widely used for decades to enhance the appearance of processed foods, beverages, and cosmetics. Among these, Tartrazine (Yellow 5, E102) is one of the most prevalent, with an estimated global production exceeding 10,000 metric tons annually, and it is commonly present in carbonated drinks, confectionery, and snack foods [1]. In the United States, the Food and Drug Administration (FDA) certifies millions of pounds of Yellow 5 each year, and children aged 4–12 have been estimated to consume 1.1–2.3 mg/kg/day [2]. Despite its ubiquity, prior studies have demonstrated that Tartrazine is associated with potential neurotoxicity, oxidative stress, and behavioral changes [3–5], and that tartrazine likely induces possible structural and functional alterations in animal models [6].
Methyl Yellow (Butter Yellow) is another food dye that is structurally related to azo dyes. It was once widely used in food products. But it was banned in the 1930s following studies that showed it was likely linked to liver tumors in rats [7–8]. Residues of azo dyes, including Methyl Yellow, have nevertheless been reported in environmental samples, suggesting ongoing potential exposure [9].
Studies have further suggested that synthetic dyes may disrupt neurotransmitter signaling or intracellular calcium regulation, potentially contributing to neurodevelopmental vulnerabilities [13–14]. Furthermore, recent reports have connected food additives with hyperactivity, attention deficits, and behavioral disturbances in children. These studies have raised significant concerns about the neurological impact of these food dyes [10–12]. As a result, the governments have begun to regulate the use of these food dyes. However, regulatory responses have varied across jurisdictions: in 2024, California prohibited six artificial dyes—including Yellow 5—from foods served in public schools [15], the European Union requires warning labels on products containing certain dyes [16], and the U.S. FDA has begun tracking industry pledges to phase out petroleum-based food dyes, with companies publishing phase-out timelines.
To define the potential neuronal toxicity of the food dyes, we used calcium imaging and laser-induced shockwave (LIS) injury [17] to evaluate whether dye exposure altered calcium signaling or injury responses. Our results show that these dyes alter neuronal injury and calcium signaling in primary culture mouse neurons. This exploratory work was intended to establish a basic framework for understanding how artificial food dyes could affect neuronal calcium dynamics and injury responses. The findings presented here are not intended as definitive evidence of hazard but rather as a starting point for future in vivo, mechanistic, and epidemiologic investigations into the potential neurological and public-health implications of synthetic food dye exposure.
2. Materials and Methods
2. 1. Cell Culture and Preparation
Primary hippocampal neurons and cortical neurons were dissociated from postnatal day 0 (P0) mouse pups and plated on 35 mm glass-bottom dishes (CELL E&G, San Diego, CA) pre-coated with 0.1% poly-L-lysine for 1 h at room temperature. Cells were initially maintained in plating medium (Neurobasal medium supplemented with 10% fetal bovine serum, 1× B27, and 1× Glutamax; Invitrogen, Carlsbad, CA). After 24 h, the medium was replaced with serum-free maintenance medium (Neurobasal, 1× B27, 1× Glutamax) to minimize glial proliferation. Cells were maintained in neuronal culture medium at 37°C in a humidified incubator containing 5% CO₂. Medium changes were performed every other day, and experiments were conducted on neurons between 4–10 days in vitro (DIV).
2. 2. Calcium Imaging and Dye Treatments
Hippocampal cells were incubated with Fluo-4 AM (4.5 µg/mL) for 35 minutes at 37°C, followed by washing with fresh medium. For viability assessment, Ethidium Homodimer III (1 µg/mL, Dead Red) was added to the medium. Cortical neurons were similarly loaded with Fluo-4 AM, and probenecid was included to reduce dye efflux.
Cells were treated with Yellow 5 (Tartrazine) or Methyl Yellow at concentrations of 50 µM or 100 µM, dissolved either in sterile water or in culture medium to evaluate solvent-dependent effects. Time-lapse fluorescence imaging was performed for 20 minutes using a Zeiss Axiovert 200 M microscope, with image acquisition every 3 seconds.
2. 3. Laser-Induced Shockwave (LIS) Injury
Mechanical injury was simulated using a laser-induced shockwave system adapted from Gomez-Godinez et al. [17]. A Coherent Flare 1030 nm laser (Spectra-Physics, Mountain View, CA) with a 1.5 ns pulse width, and 450 μJ pulse energy was used to generate shockwaves. Beam power was attenuated using a rotating optical polarizer mounted on a motorized stage (Newport, Irvine, CA). A mechanical shutter (Vincent Associates, Rochester, NY) permitted 1–2 laser pulses with a duty cycle of 10–15 ms.
The laser beam was expanded to fill the back aperture of a 20× NA 0.6 Zeiss objective on a 200 M Zeiss microscope. The focal point was positioned approximately 10 μm above the substrate. Measured power at the objective was 200–220 μW. A Green Fluorescent Protein (GFP) filter set (Zeiss, Germany) and a Red Fluorescent Protein (RFG) was mounted on the microscope filter turret (Zeiss, Germany) and controlled via Micromanager.
Hippocampal and cortical neurons were exposed to LIS under both control and dye-treated conditions. Calcium responses before and after shockwave application were recorded by the ORCA-Flash4.0 V2 Hamamatsu CMOS camera. Cell death was assessed by Dead Red staining and phase imaging collected through the same camera. Images from the three channels were analyzed in the ImageJ software and used to quantify the radius of cell detachment.
3. Results
3.1. Effects of Yellow 5 and Methyl Yellow on Hippocampal Neuron Calcium Signalling
As detailed in Section 2.2, Fluo-4 AM–loaded hippocampal neurons were imaged to quantify early (2.5 min) and sustained (20 min) calcium responses under control and dye conditions, as shown in Figures 1 and 2 respectively. Control treatments (water or DMSO) produced a modest fluorescence increase of ~32% at 2.5 minutes, whereas 50 μM Yellow 5 elicited a markedly larger rise (~90%), and 100 μM Methyl Yellow produced the greatest increase (~119%) (Figure 1A). Notably, 100 μM Yellow 5 yielded a smaller early response (~51%) than 50 μM, indicating a non-linear concentration dependence.
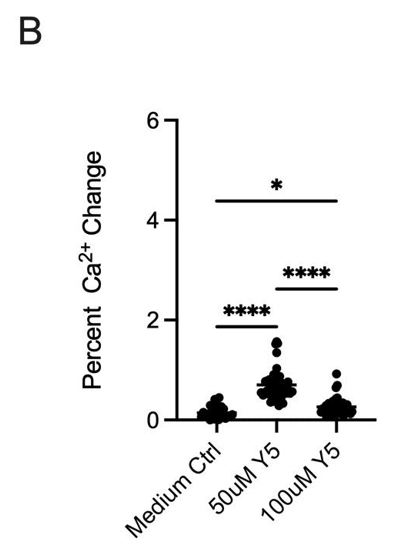
To exclude effects related to dissolving Yellow 5 in water, the dye was also prepared in culture medium. As shown in Figure 1B, medium alone produced a mean ~23% elevation (observed in 16/50 cells), while 50 μM Yellow 5 in medium induced a ~70% rise (44/50 cells). Consistent with the water condition, 100 μM Yellow 5 again produced a lower increase (~34%) than 50 μM. These findings indicate that dye-evoked calcium elevations were robust and not attributable to the diluent. The attenuated signal at 100 μM Yellow 5 may reflect concentration-dependent phenomena (e.g., aggregation/self-quenching, interference with Fluo-4 fluorescence, or reduced cellular uptake).
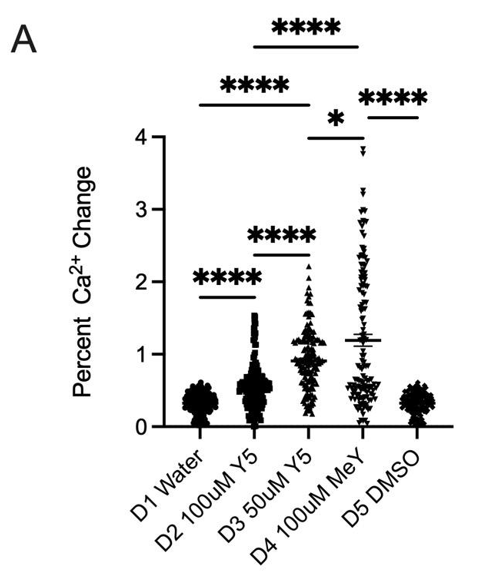
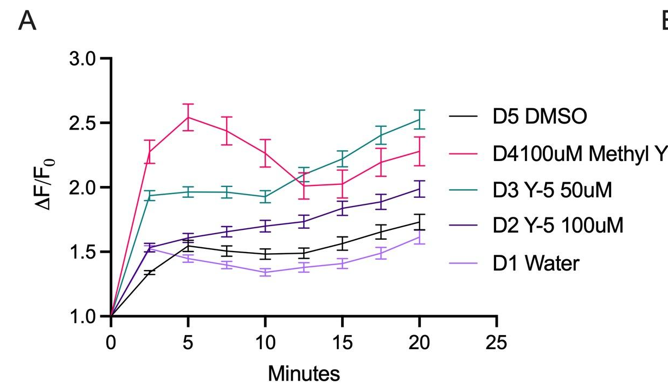
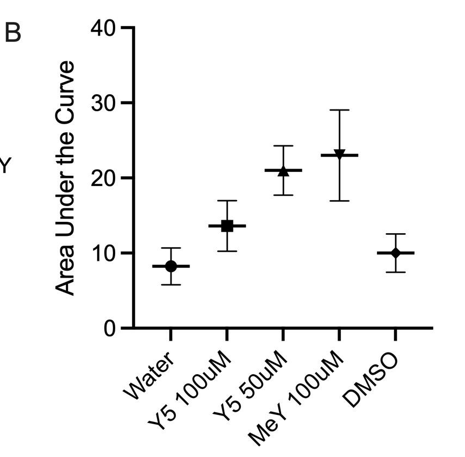
Across the full 20-minute imaging window, dye-induced elevations persisted, consistent with prolonged dysregulation of intracellular calcium (Figure 2A). Area-under-the-curve quantification confirmed substantially greater cumulative calcium displacement for 50 μM Yellow 5 and 100 μM Methyl Yellow compared with controls. Methyl Yellow exhibited the most pronounced calcium displacement/AUC (Figure 2B). Together, these data support the conclusion that both dyes disrupted calcium homeostasis in hippocampal neurons—an essential regulator of neuronal survival and signaling integrity—and motivated follow-up controls to resolve the paradoxical reduction observed at 100 μM Yellow 5.
3.2. Impact of Yellow 5 on Hippocampal Neuron Calcium Injury Responses Following Laser-Induced Shockwave
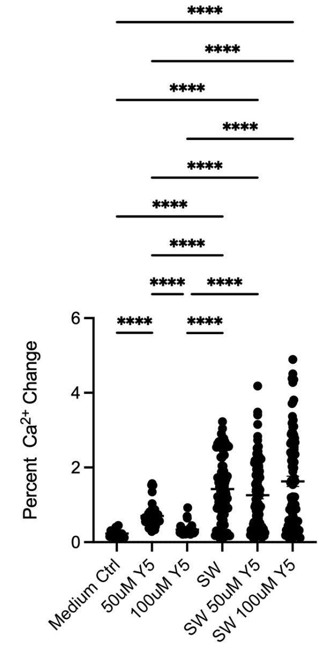
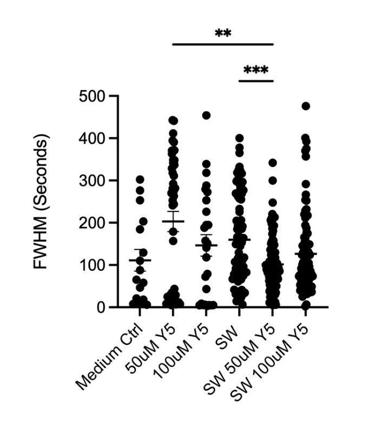
Laser-induced shockwave (LIS) was applied to hippocampal neuron cultures to assess whether Yellow 5 altered calcium responses to acute mechanical injury. Three features of the transient were quantified: peak increase (ΔF/F₀), duration (full width at half maximum, FWHM), and total calcium load (area under the curve, AUC).
- Peak amplitude: LIS elicited a significantly larger percentage increase in fluorescence than exposure to Yellow 5 alone (Figure 3A). Statistical significance was denoted in the figure with asterisks (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001).
- Duration: The FWHM of the LIS-evoked spike was shorter in the presence of 50 μM Yellow 5 than for LIS in medium alone, indicating that the elevation resolved more rapidly when Yellow 5 was present (Figure 3B).
- Total calcium displacement: Consistent with the amplitude findings, the area under the curve (AUC) was greater for LIS than for dye addition without injury. These findings confirm higher cumulative calcium displacement under mechanical insult. Notably, AUC was smaller for LIS in 50 μM Yellow 5 than for LIS medium control. Suggesting that Y5 is affecting calcium modulation (Figure 3C).
From the above three metrics, the overall pattern was consistent with the notion that Yellow 5 did not prevent the initial injury-evoked surge but altered its temporal profile, yielding a shorter-lived transient and a lower cumulative calcium burden. These effects could have arisen from (i)biological modulation of injury-response pathways (e.g., changes in membrane conductance or calcium extrusion/sequestration) and/or (ii) measurement-related interactions (e.g., concentration-dependent effects on Fluo-4 dynamic range or nonspecific binding to cellular structures). Additional controls (in situ Fluo-4 calibration, inner-filter/self-quenching tests, and pharmacological dissection of influx vs. extrusion) may help differentiate these possibilities.
(A)
(B)

(C)
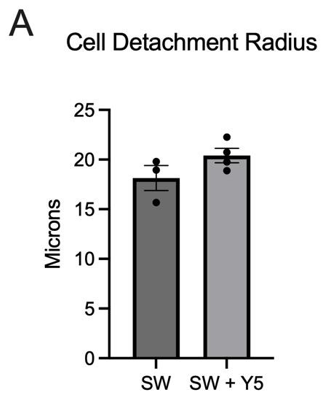
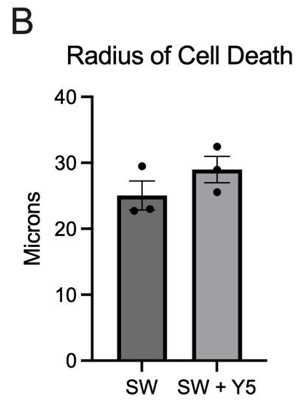
3. 3. Impact of Yellow 5 on Hippocampal Neuronal Cell Death Following Laser-Induced Shockwave
Cell death and detachment following laser-induced shockwave were assessed by Dead Red staining and brightfield imaging. The radial extent of injury was quantified as the mean radius (μm) of Dead Red–positive cells and/or detached regions. Cultures exposed to LIS alone exhibited a smaller mean radius (18.1 μm) than cultures exposed to SW in the presence of Yellow 5 (20.92 μm), indicating a trend toward greater spatial spread of injury when Yellow 5 was present (Figure 4). No statistically significant difference was detected at the current sample size, although the mean value appeared higher with Yellow 5, consistent with increased vulnerability under combined dye and mechanical insult.
These findings aligned with the calcium-imaging results, in which injury-evoked dynamics were altered by Yellow 5, and suggested that the dye may have modulated downstream injury processes (e.g., membrane compromise or intracellular signaling that governs death pathways). Additional replicates with powered sample sizes, orthogonal viability readouts, and time-resolved injury mapping are necessary to determine whether the observed trend reflects a reproducible increase in cell-death radius under Yellow 5 exposure.
3.4 Effects of Yellow 5 addition on calcium responses of primary cortical cultures
To extend the injury analysis beyond hippocampal neurons, four dishes of mixed(neurons and glia) mouse cortical culture were examined: water control (D1), 100 μM Yellow 5 in water (D2), medium control, DMSO (D3), and 100 μM Yellow 5 in medium (D4) (Figure 5A–C). The mouse cortical cells were also loaded with Fluo-4 Calcium dye,) and imaged as 600 time-lapse frames acquired every 3 seconds. Calcium trajectories were extracted by a MATLAB algorithm and summarized as peak values (ΔF/F₀), full width at half maximum (FWHM), and area under the curve (AUC) on Figure (5A–B).
Relative to the corresponding water control, 100 μM Yellow 5 in water yielded smaller and shorter calcium spikes following addition of food dye, indicating a faster injury-evoked calcium response and recovery. A significant reduction in FWHM was observed between water control and Yellow 5 in water, consistent with accelerated decay kinetics under dye exposure.
The AUC analysis revealed solvent-dependent effects of Yellow 5: when dissolved in medium, Yellow 5 produced a lower cumulative calcium displacement than medium control. When dissolved in water, Yellow 5 produced a higher cumulative displacement than water control. These divergent patterns suggested that both dye presence and dissolution medium influenced the overall calcium burden after mechanical injury.
In summary, the cortical culture data indicated that Yellow 5 triggered calcium increases that differed from solvent additions. The calcium increases caused by the addition of Y5 were shorter than those caused by solvent additions (Figure 5B). The findings were consistent with a model in which dye exposure affects calcium modulation (Figure 5A–C).
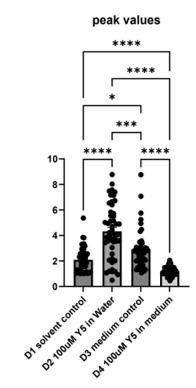
(A)

(B)
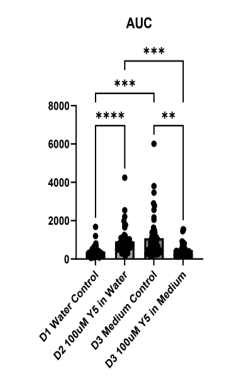
(C)
4. Conclusion
This study provided an initial examination of how artificial food dyes influenced neuronal calcium signaling and injury responses in primary mouse neurons. In hippocampal cultures, exposure to Yellow 5 and Methyl Yellow was associated with exaggerated early calcium elevations at 2.5 minutes and sustained increases over 20 minutes, with Methyl Yellow producing the largest effects. A paradoxical attenuation at 100 μM Yellow 5 relative to 50 μM was observed across solvent conditions, suggesting concentration-dependent phenomena such as dye aggregation, self-quenching, altered Fluo-4 performance, or changes in uptake. Under laser-induced shockwave (LIS), injury-evoked calcium transients remained robust; however, the presence of 50 μM Yellow 5 shortened the transient (reduced FWHM) and lowered the cumulative calcium load (AUC) relative to LIS in medium, indicating modulation of the temporal profile rather than prevention of the surge. Cell-level outcomes assessed by Dead Red and brightfield imaging showed a larger mean radius of detachment/death in LIS with Yellow 5 than in LIS alone, although the difference did not reach statistical significance at the current sample size, suggesting a trend toward increased vulnerability under combined dye exposure and mechanical stress.
Taken together, these findings indicate that Yellow 5 perturbed calcium homeostasis and modified injury-evoked signalling dynamics in hippocampal neurons, while Methyl Yellow served as a comparator with consistently stronger dysregulatory effects. The net impact of Yellow 5 appeared complex: dye exposure heightened calcium responsiveness in uninjured conditions, yet during LIS the transient became shorter and the cumulative load decreased, pointing to possible biological modulation of recovery kinetics and/or measurement interactions with the calcium indicator.
Several limitations should be acknowledged. The reliance on a single chemical indicator (Fluo-4) without in situ calibration or complementary reporters may have introduced measurement bias; sample sizes for cell-death assays limited statistical power; and only a narrow concentration range and acute exposure window were evaluated in vitro. Future work should include dose–response studies spanning human-relevant exposures, multi-indicator validation (e.g., ratiometric dyes and genetically encoded indicators), electrophysiology to link calcium dynamics with excitability, pharmacological dissection of sources and sinks (plasma membrane channels, ER, mitochondria, extrusion/sequestration), and expanded viability endpoints (e.g., calcein AM/propidium iodide, caspase activation) with powered replication.
The food dye concentrations utilized in this study are approximately 5-10x higher than what has been estimated to make it into the bloodstream after ingestion [18]. However, the amounts of dye estimated to be found in food and beverages range from 10-300mg/L(18.7- 561 μM), which are within the range of the concentrations we tested and potentially the range to which the gastrointestinal tract is being exposed to [19]. Additionally, a concentration of 131 μM tartrazine has been found to lead to DNA damage in human leukocytes [20]. Therefore, our selected concentrations of 50 and 100 μM seemed physiologically relevant towards our understanding of how artificial food dyes may be affecting calcium modulation at the cellular level. Nevertheless, studies with lower concentrations should also be done to determine if similar disruptions in calcium signalling are observed at concentrations estimated to be found in the bloodstream. Additionally, longer exposures to food dye should be conducted to determine the potential long-term impacts on calcium signalling. Future investigations should also include differences in calcium responses in neurons vs glial cells in cortical cultures subjected to LIS.
Within these constraints, the data supported a working model in which artificial food dyes—particularly Methyl Yellow and, under specific conditions, Yellow 5—altered neuronal calcium signaling and influenced cellular responses to mechanical insult. These observations justified further investigation into mechanistic underpinnings and potential neurodevelopmental implications, while avoiding over-generalization beyond the preliminary in vitro context presented here.
5. Acknowledgements
This work was supported by a generous gift from Beckman Laser Institute Inc. to LS & VGG. The high school students (†) participated in the UCSD IEM OPALS internship. Special thanks to Dr. Shu Chien from UCSD Bioengineering, Dr. Lizhu Chen from CorDx Inc., Dr. Xinhua Zheng, and David & Leslie Lee, Mingwei He & Wen Shi for their generous donations.
References
[1] U.S. Food and Drug Administration, Certified Color Additives in Food: Annual Summary, 2022. Available: View Article
[2] J. Smith, "Dietary exposure to Tartrazine in U.S. children," Journal of Food Science, vol. 88, no. 3, pp. 1021-1030, 2023.
[3] A. Lee and B. Chen, "Behavioral effects of artificial coloring in children," Pediatrics, vol. 29, no. 3, pp. 289-303, 2021.
[4] S. Robinson, "Food additive toxicity studies: A review," Toxicology Reports, vol. 6, pp. 728-742, 2020.
[5] M. Patel and R. Singh, "The role of calcium signaling in neurotoxicity of food additives," Journal of Neurochemistry, vol. 146, no. 1, pp. 10-22, 2019.
[6] L. Khayyat, A. Essawy, J. Sorour, and A. Soffar, "Tartrazine induces structural and functional aberrations and genotoxic effects in vivo," PeerJ, vol. 5, article e3041, 2017, doi: 10.7717/peerj.3041. PubMed+2PeerJ+2 View Article
[7] J. A. Miller and E. C. Miller, "The carcinogenicity of Methyl Yellow in rats," Journal of the National Cancer Institute, vol. 8, no. 2, pp. 123-130, 1947.
[8] Environmental Persistence of Azo Dyes. (2021). [Online]. Available: View Article
[9] A. Tkaczyk, K. Mitrowska, and A. Posyniak, "Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review," Science of the Total Environment, vol. 717, article 137222, 2020, doi: 10.1016/j.scitotenv.2020.137222. PubMed+2ResearchGate+2 View Article
[10] M. J. Stevens, "Synthetic food colors and hyperactivity: A meta-analysis," Journal of Developmental & Behavioral Pediatrics, vol. 35, no. 3, pp. 179-186, 2014.
[11] D. McCann, A. Barrett, A. Cooper, D. Crumpler, L. Dalen, K. Grimshaw, E. Kitchin, K. Lok, L. Porteous, E. Prince, E. Sonuga-Barke, J. O. Warner, and J. Stevenson, "Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: A randomised, double-blinded, placebo-controlled trial," The Lancet, vol. 370, no. 9598, pp. 1560-1567, 2007. View Article
[12] B. Bateman, J. O. Warner, E. Hutchinson, T. Dean, P. Rowlandson, C. Gant, J. Grundy, C. Fitzgerald, and J. Stevenson, "The effects of a double-blind, placebo-controlled, artificial food colourings and benzoate preservative challenge on hyperactivity in a general population sample of preschool children," Archives of Disease in Childhood, vol. 89, no. 6, pp. 506-511, 2004. View Article
[13] I. Buka, A. Osornio-Vargas, and B. Clark, "Food additives, essential nutrients and neurodevelopmental behavioural disorders in children: A brief review," Journal of Attention Disorders, vol. 23, no. 5, pp. 435-442, 2019.
[14] E. H. Wong and I. Kwon, "Xanthene food dye as a modulator of Alzheimer's disease amyloid-β peptide aggregation and the associated impaired neuronal cell function," Journal of Neurochemistry, vol. 158, no. 4, pp. 912-925, 2021.
[15] "California is banning artificial food dyes in school snacks and drinks. Here's what the science says," CalMatters, Sept. 30, 2024. [Online]. Available: View Article
[16] Y. F. Sasaki, S. Kawaguchi, A. Kamaya, M. Ohshita, K. Kabasawa, K. Iwama, K. Taniguchi, and S. Tsuda, "The comet assay with eight mouse organs: Results with 39 currently used food additives," Mutation Research, vol. 519, no. 1-2, pp. 103-119, 2002. View Article
[17] V. Gomez-Godinez, D. Preece, L. Shi, N. Khatibzadeh, D. Rosales, Y. Pan, L. Lei, Y. Wang, and M. W. Berns, "Laser-induced shockwave paired with FRET: A method to study cell signaling," Microscopy Research and Technique, vol. 78, pp. 195-199, 2015. View Article
[18]L-L. Jiang, K. Li, D-L. Yan, M-F. Yang, L. Ma, L-Z. Xie. "Toxicity Assessment of 4 Azo Dyes in Zebrafish Embryos." International Journal of Toxicology.2020;39(2):115-123 View Article
[19] D.L. Doell, D.E Folmer, H.S. Lee, K.M. Butts, S.E. Carberry. "Exposure estimate for FD&C colour additives for the US population." Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2016 May;33(5):782-97. View Article
[20] J.M. Floriano, E. da Rosa, Q.D.F. do Amaral, L. Zuravski, P.E.E. Chaves, M.M. Machado, L.F.S de Oliveira. Is tartrazine really safe? In silico and ex vivo toxicological studies in human leukocytes: a question of dose. Toxicol Res (Camb). 2018 Jul 20;7(6):1128-1134. doi: 10.1039/c8tx00034d. PMID: 30510682; PMCID: PMC6220720. View Article

