Volume 11 - Year 2024 - Pages 25-32
DOI: 10.11159/jbeb.2024.004
EGFR Tyrosine Kinase Inhibitors: A Comprehensive Analysis of Adverse Events, Costs, and Prescribing Patterns
Shravani Gote1
1West Windsor Plainsboro High School South, Department of Biology
346 Clarksville Rd, Princeton Junction, NJ 08550
25sg1185@wwprsd.org
Abstract - Lung cancer is the leading cause of cancer death worldwide among men and women, and non-small cell lung cancer (NSCLC) accounts for 85% of all diagnosed lung cancer cases in the United States. Mutations in the epidermal growth factor receptor (EGFR) gene cause continuous activation of the EGFR and its downstream signalling pathways involving cell proliferation and apoptosis, leading to excess cell division and tumor growth in NSCLC. EGFR Tyrosine Kinase Inhibitors (TKIs), a targeted therapy for NSCLC patients with EGFR mutations, bind to the ATP-binding site of EGFR and prevent the activation of downstream signalling pathways and slow or stop the growth of cancerous cells. The objective of this study is to evaluate the performance and the cost of three TKIs - Gefitinib, Afatinib, and Erlotinib - within the real world setting. This evaluation was conducted by assessing trends in reported adverse events, costs, and prescriptions using data collected from the FDA Adverse Event Reporting System (FAERS) and the Medicare Part D Database. This work is novel because it is the first to use both FAERS and the Medicare Part D Database to track trends in the aforementioned variables over multiple years, allowing for a comprehensive analysis of the real world effectiveness and economic impact of the three TKIs. From 2001 to 2024, Gefitinib recorded 8,543 adverse events, Erlotinib recorded 14,725, and Afatinib recorded 6,193. The three TKI treatments cost American patients approximately $2.8 billion in total. Analysis of the data revealed that the adverse event rate was higher among the first generation TKIs in comparison to their second generation counterpart and highlighted the significant financial burden TKI treatment puts on patients. Future work should focus on improving the safety of TKIs by monitoring their adverse events and increasing the affordability of these life saving drugs.
Keywords: EGFR tyrosine kinase inhibitors (TKIs), Epidermal Growth factor receptor (EGFR), Adverse Events Profile, non-small cell lung cancer
© Copyright 2024 Authors This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2024-07-23
Date Revised: 2024-09-20
Date Accepted: 2024-10-01
Date Published: 2024-10-08
1. Introduction
Lung cancer remains the leading cause of cancer-related deaths worldwide among both men and women [1]. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of diagnosed lung cancer cases in the United States. NSCLC develops slowly, often resulting in delayed symptom onset until the cancer has advanced significantly. There are three main types of NSCLC: adenocarcinoma, squamous cell lung cancer, and large-cell undifferentiated carcinoma, each originating in different cell types within the lung [2].
Treatment options for NSCLC depend largely on the stage at diagnosis, which is determined by tumor size, location, lymph node involvement, and metastasis. Unfortunately, due to the disease's insidious progression, the majority of patients (66%) are diagnosed at advanced stages (III and IV), limiting curative surgical options and necessitating alternative treatment approaches [3-5].
In recent years, targeted therapies have emerged as a promising approach for treating NSCLC, particularly for patients with specific genetic mutations. One significant target is the Epidermal Growth Factor Receptor (EGFR), a tyrosine kinase receptor involved in critical cellular processes. Mutations in EGFR, present in up to 25% of NSCLC patients, can lead to continuous activation of signalling pathways, resulting in uncontrolled cell proliferation and tumor formation [6].
EGFR Tyrosine Kinase Inhibitors (TKIs) have been developed to address this issue by binding to the ATP-binding site of EGFR and inhibiting its activity. Three such drugs - Gefitinib (Iressa), Erlotinib (Tarceva), and Afatinib (Gilotrif) - have received FDA approval for treating advanced NSCLC. However, the approval history and efficacy of these drugs have been complex, with changes in approved indications based on clinical trial results and the identification of specific patient populations that benefit most from these treatments.
In May 2003, Gefitinib got accelerated approval by the FDA as a monotherapy treatment for patients with locally advanced or metastatic NSCLC after failure of both platinum-based and docetaxel chemotherapies. Under accelerated approval, AstraZeneca, the company that manufactures Gefitinib, had to conduct additional clinical trials to prove that Gefitinib improves NSCLC patient outcomes. Multiple trials failed to prove clinical benefit and as a result in September 2011, AstraZeneca withdrew Gefitinib from the market. In July 2015, the FDA approved Gefitinib for treatment, but for a different population - those who had the EGFR mutation and had not been previously treated [7]. This approval was supported by various studies that showed Gefitinib improved outcomes for NSCLC patients with the EGFR mutation, one of which was a clinical trial containing 106 patients that showed a 50% response rate in untreated patients with the EGFR mutation. This meant that half of these patients’ cancers had either gone away or shrunk. Four patients from the trial stopped Gefitinib treatment due to side effects and the most common ones included Rash, Diarrhoea, Vomiting, Asthenia, Cough, Dry skin, Nausea, and Decreased appetite [8]. In another randomized clinical trial, called IPass, among the patients who had the EGFR mutation, those treated with Gefitinib had longer progression-free survival than those who were treated with standard chemotherapy [9].
In November 2004, FDA approved Erlotinib for treatment of patients with locally advanced or metastatic NSCLC after failure of at least one prior chemotherapy regimen [10] . Five years later in 2009, Erlotinib’s manufacturers GeneTech and OSI wanted the FDA to expand Erlotinib’s approval to include cancer patients who are less sick and have been helped by chemotherapy. The FDA’s Oncologic Drugs Advisory Committee, consisting of an external panel of cancer experts, voted 12 to 1 that the FDA should not expand Erlotinib’s approval. Despite the committee’s vote, the FDA went through with this expansion and requested that further studies be conducted into whether the drug improves patient outcomes [11]. In the IUNO study, a randomized, double-blind, placebo-controlled trial consisting of patients without the EGFR mutation, Erlotinib did not show superior progression-free survival outcomes over the placebo [12]. As a result in 2016 the FDA limited Erlotinib’s usage to only NSCLC patients with the EGFR mutation. In 2013, The FDA approved Afatinib to treat patients with metastatic NSCLC who have EGFR exon 19 deletions or exon 21(L858R) substitution. Three years later, the FDA expanded the drug’s approval to treat metastatic squamous NSCLC after platinum-based chemotherapy. In 2018, Afatinib was approved to be used in the first line treatment of patients with metastatic NSCLC [13].
The different TKIs can be classified into generations based on their chemical structure and function. Gefitinib and Erlotinib are classified as first generation TKIs. They bind reversibly to the protein tyrosine kinase (PTK) domain of the EGFR through noncovalent interactions such as hydrogen-bonding, electrostatic and hydrophobic interactions, inhibiting ATP from binding to the PTK, which prevents the activation of EGFR and its associated cellular pathways. On the other hand, Afatinib is classified as a second generation EGFR inhibitor. Afatinib can irreversibly inhibit EGFR activity because it binds covalently with the EGFR and this ability gives it an advantage over its first generation counterparts [14]. While these TKIs have shown promise in improving outcomes for NSCLC patients with EGFR mutations, there is a need for comprehensive analysis of their real-world performance, adverse events, and economic impact. This study aims to address this gap by investigating trends in adverse events, costs, and prescriptions associated with Gefitinib, Afatinib, and Erlotinib. By utilizing data from the FDA Adverse Event Reporting System (FAERS) and the Medicare Part D Database, this research provides a novel approach to understanding both the clinical and economic aspects of these important targeted therapies for NSCLC.
3. Methods
The FDA Adverse Event Reporting System (FAERS) was used to track the adverse events of the 3 TKIs investigated in this study - Gefitinib, Erlotinib, and Afatinib [15]. FAERS consists of both mandatory reports submitted by drug manufacturers and required by the FDA, detailing adverse events that have been associated with manufacturers’ drugs, and voluntary reports, submitted by consumers and healthcare professionals. For each EGFR inhibitor, the generic name was used as the search term - Gefitinib, Erlotinib and Afatinib - and the data found under the Demographics tab were used to record the number of adverse event cases from 2001 to 2024. The total number of adverse events per drug from 2001 to 2024 can also be found under Demographics next to the Totals column in the FAERS.
The Medicare Part D Database was used to analyse prescribing trends as it includes all prescriptions filled for patients taking FDA approved drugs under the Medicare part D plan [16]. For each EGFR inhibitor, the generic name was used as the search term - Gefitinib, Erlotinib and Afatinib. To determine the total number of 30-day fills per year from 2013 to 2022 for each drug, the data under Tot_30day_Fills at the National level was used. To determine the total cost for each drug from 2013 to 2022, the data under Tot_Drug_Cst at the National level was used. The cost per 30-day fill from 2013 to 2022 for each drug was calculated by dividing total cost per year by the total number of 30-day fills. The rate of adverse events per 30-day fill annually was calculated by dividing total number of adverse events by number of 30-day fills.
4. Results
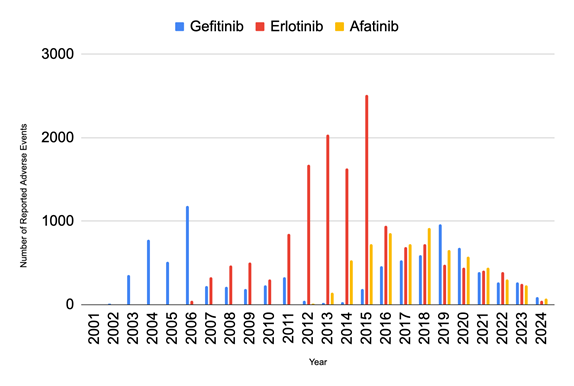
Analyzing the annual trends in reported adverse events for TKIs (Figure 1), it is clear that Erlotinib had the largest peak in adverse events out of the 3 drugs. Erlotinib’s adverse events peaked in 2015 at 2,511 cases and then dropped drastically by 62.28% to 949 cases in the following year. The number of adverse events associated with Erlotinib decreased by 73.87% over the seven years after 2016, reaching a low of 248 cases in 2023. Gefitinib’s adverse events reached their highest in 2006 with 1,180 cases and then decreased significantly by 80.85% to 226 in 2007. They peaked again in 2019 at 480 cases and gradually decreased by 48.33% over the next four years, reaching a low of 248 cases in 2023. The peak of the reported adverse events for Afatinib,at 921 cases in 2018, was lower than that of its counterparts. Like Gefitinib and Erlotinib, Afatinib’s adverse events also decreased and reached a low in 2023, with 233 cases for Afatinib.
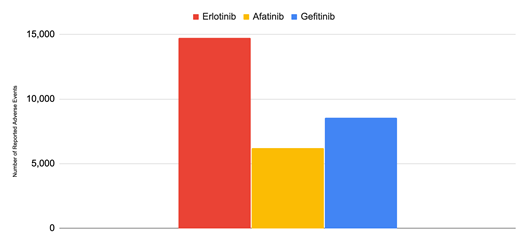
From 2001 to 2024, Gefitinib recorded a total of 8,543 adverse events, Erlotinib recorded 14,725, and Afatinib recorded 6,193 (Figure 2). Erlotinib had substantially higher amounts of adverse events compared to its counterparts, with more than double the adverse events of Afatinib and nearly 1.5 times the adverse events of Gefitinib. Taken together, there were 29,461 reported adverse events from these 3 TKIs from 2001 to 2024. The adverse events for each of the first generation TKIs, Gefitinib and Erlotinib, were significantly greater than the adverse events for the second generation TKI Afatinib. Among the most common adverse events were general disorders and administrative site conditions such as Death, Drug Resistance, Fatigue, as well as gastrointestinal disorders such as Diarrhea, Nausea, Vomiting, Stomatitis, Abdominal Pain, Constipation, and Dysphagia.
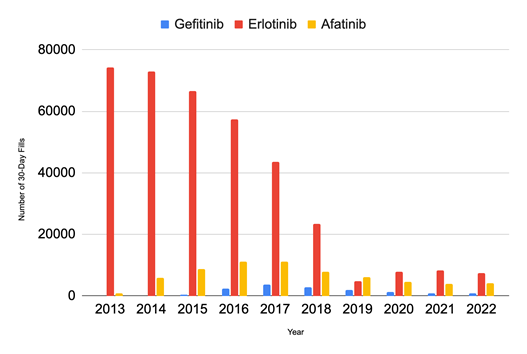
With regard to the trends in the number of 30-day fills annually from 2013 to 2022 (shown in Figure 3), Erlotinib has the greatest number of 30-Day fills per year, peaking at 74,318 in 2013 and subsequently declining by 68.45% to 23,428 in 2018. Between 2018 and 2019, there was a notable decrease of 79.41% and the fills reached their lowest amount of 4,822. The number of fills for Gefitinib was greatest in 2018 when there were 2,687 fills and they continued to decrease until 2022. The number of fills for Afatinib reached their greatest amount in 2017 with 11,081 cases, experiencing a gradual 62.39% decrease over the following four years to reach a minimum of 4,166 in 2022. The total number of 30-day fills from 2013 to 2022 for Erlotinib was 366,329.40, nearly 26 times greater than that of Gefitinib which had 14,049.80 fills, and 5 times greater than that of Afatinib which had 64,335 fills.
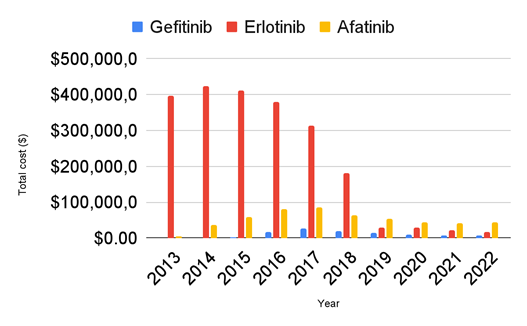
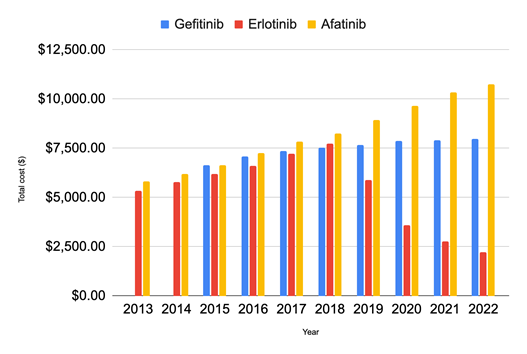
In total, from 2013 to 2022, these three TKIs cost $2,821,040,347.01 (Figure 4). The total cost of Erlotinib was greatest in 2013 at $422,596,683.23. The cost decreased gradually over the following 5 years by 57.11%, reaching a low of $181,251,150.19 in 2018. Between 2018 and 2019, there was a significant decrease in the total cost, nearly 84.31%. Gefitinib’s cost was at its highest of $26,395,400.32 in 2017 and decreased by 74.89% to $6,625,714.25 in 2022. Afatinib’s cost was at its greatest in 2017, reaching $86,639,174.81 and decreased by 48.31% to $44,799,471.74 in 2022. The total cost of Erlotinib from 2013 to 2022 was $2,199,766,158.53, twenty times greater than the total cost of Gefitinib which was $105,125,010.88 and approximately four times greater than the total cost of Afatinib which was $516,149,177.60.
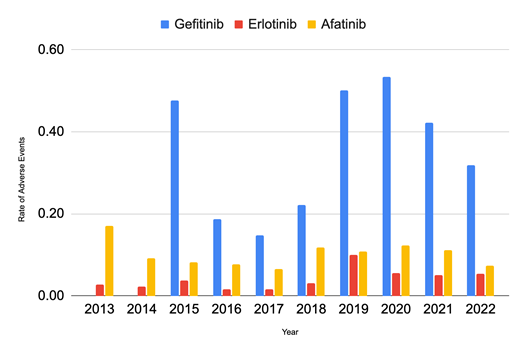
Afatinib’s cost per fill increased by 85.41% from 2013 to 2022 (Figure 5), reaching $10,751.53 in 2022, a much greater rate of increase than that of Gefitinib, which increased by 19.96% over the nine years and cost $7,973.18 in 2022. While Erlotinib showed an increasing trend until 2018, when it cost $7,736.49, there was a sudden 23.85% drop in cost in 2019, and it continued to decrease over the next 3 years by 62.23% and was priced at $2,223.86 in 2022. The rate of adverse events (Figure 6) in Gefitinib is significantly greater than that of Erlotinib and Afatinib from 2013 to 2022. At its peak in 2020, the adverse rate for Gefitinib was nine times that of Erlotinib and approximately four times that of Afatinib.
5. Discussion
This study is novel in its use of a comprehensive longitudinal analysis of adverse event data over nearly two decades (2001-2024), paired with Medicare Part D prescription patterns, to assess the impact of regulatory decisions on drug safety and patient outcomes. The adverse event trends reflect and inform FDA and drug manufacturer decisions to expand or restrict the approved indications for TKIs. Erlotinib showed an increase in adverse events after 2009, because the FDA expanded the drug’s approval in 2009 to treat cancer patients who are less sick and have been helped by chemotherapy. As the population of patients being treated with the prescription grew, so did the amount of adverse events. The reverse occurred in 2016 after the FDA limited the approval of Erlotinib to treat only NSCLC patients who have the EGFR mutation, and this decrease in the patient pool is reflected by the 62.28% decrease in adverse events during that year. Similarly, after clinical trials failed to prove that Gefitinib improves patient outcomes and it was removed from the market in 2011, there was a decrease in adverse events. The few adverse events between 2011 and 2014, a time during which the FDA did not approve Gefitinib and it was off the market, are likely due to physicians prescribing the drug off-label. The adverse events began to rise again after 2015 because Gefitinib was approved again by the FDA to be used as a treatment for those with the EGFR mutation and who had not been previously treated. Afatinib was first approved by FDA in 2013 and its approval was expanded later in 2018 when it was used in the first line treatment of patients with metastatic NSCLC. The peak of adverse events for Afatinib in 2018 corresponds with the drug becoming available as a treatment option to a greater patient population.
The novelty of this study also lies in its analysis of adverse event rates in the context of accelerated drug approvals. The FDA speeds up the approval process for TKIs such as Gefitinib because many cancer patients do not have any other treatment options. This accelerated process has a lot of benefits - patients can access treatment earlier and the clinical trials are shorter and less expensive. However, the issue with accelerated approval is that the drug can reach the market before physicians and scientists are aware of all of its potential side effects and are 100% confident that the drug improves patient outcomes. The shorter clinical trial is efficient and cost-effective but is unable to show the long term effectiveness and safety of a drug. As a result many clinical trials are conducted after the drugs’ approval; for all three TKIs in the current study, the FDA used data from trials conducted post-approval to restrict or expand the availability of the drug to certain patients. These decisions are also informed by the adverse events reported by physicians and consumers that highlight the wide range of side effects the drug can bring about that were previously unknown.
In total from 2001 to 2024, Erlotinib recorded nearly twice the number of events of Afatinib and 1.5x that of Gefitinib. Based on the number of 30-day fills, Elrotinib was prescribed significantly more often to those under the Medicare Part D plan, nearly 26x more often than Gefitinib and 5x more than Afatinib. Because Erlotinib is prescribed significantly more often than its counterparts, it makes sense that the adverse events for Erlotinib are so frequent, so the adverse event rate is a more informative metric. Gefitinib’s rate of adverse events is significantly greater than that of Afatinib and Erlotinib. At its peak in 2020, the adverse event rate for Gefitinib was nine times that of Erlotinib and approximately four times that of Afatinib. The peak in the adverse event rate in 2020 for all three TKIs is likely correlated with coronavirus disease 2019 (COVID-19).
Afatinib has a lower adverse event rate than Gefitinib and Erlotinib. Afatinib has better patient outcomes over its two counterparts because, over time, cancer cells can develop resistance to first generation TKIs such as Gefitinib and Erlotinib resulting in treatment becoming ineffective. This aligns with previous studies that indicate that first-generation TKIs tend to have a broader toxicity profile [17 - 18]. Since Afatinib is a second generation EGFR inhibitor, it can overcome resistance and remain effective in cancer treatment [19]. Despite Erlotinib and Gefitinib both being first generation TKIs, Erlotinib had a lower adverse event rate than Gefitinib. This is because Erlotinib was introduced to the market as a first generation TKI after Gefitinib, therefore, it's likely that chemical modifications were made by Erlotinib’s manufacturer to ensure it had a better safety profile than the drug that came before it which was Gefitinib.
The adverse events reflect the need for vigilant monitoring of patients undergoing TKI therapy to mitigate the risks associated with these adverse events.. For example, a study reported that afatinib was associated with a higher risk of gastrointestinal toxicities, while Erlotinib and Gefitinib were linked to skin-related adverse events [17]. Furthermore, the incidence of serious infections has been documented to be higher in patients treated with EGFR TKIs, particularly in those receiving first-generation TKIs[20].
In total, the three TKIs cost Americans $2.8 billion from 2013-2022. On average, across all three drugs from 2013-2022, one 30-day fill of the drug cost roughly $7,000. NSCLC and its treatment itself takes a huge emotional toll, but the staggering cost places a debilitating financial burden on individuals, families and American society.
This economic burden is exacerbated by the need for continuous treatment and management of adverse events, which can lead to increased healthcare utilisation [21]. A retrospective study indicated that patients receiving first-line EGFR TKIs often experience significant out-of-pocket expenses, which can deter treatment adherence [21]. Moreover, the financial toxicity associated with these therapies is compounded by the high incidence of adverse events, which necessitates additional medical interventions (Nieva et al., 2022). The high costs associated with these therapies may limit access for some patients, particularly those with inadequate insurance coverage]. This financial barrier underscores the necessity for policy interventions aimed at reducing the cost of cancer therapies, thereby improving access to life-saving treatments for patients with EGFR-mutated NSCLC [22].
Future research should focus on optimizing the safety and efficacy of EGFR TKIs while addressing the economic challenges associated with their use. Strategies may include the development of biomarkers to predict adverse events and treatment responses, which could facilitate personalized treatment approaches [23]. Additionally, ongoing efforts to improve the affordability of these therapies through policy changes and innovative pricing models are essential to enhance patient access and adherence [22].
Limitations of this study include potential inaccuracies in the FAERS database, because a reported adverse event may not necessarily have been caused by the drug, the report of the adverse event may be incomplete, there is no way to verify that an adverse event has occurred, and there may be duplicate reports. While vast, the Medicare Part D is limited in that it is restricted to those on Medicare Part D healthcare (those over 65 or those with qualifying disabilities) and does not list every single prescription written for a given drug.
6. Conclusion
This study investigated the adverse event profiles, cost, and prescribing trends associated with three TKIs, Gefiitnib, Erlotinib and Afatinib, using data from FAERS and the Medicare Part D Database. The findings provide a novel comparison of adverse event rates per 30-day fill between first- and second-generation TKIs, demonstrating Gefitinib’s higher adverse event rate and Afatinib’s lower rate, likely due to its ability to overcome drug resistance, unlike its first generation counterparts. The adverse event rates fluctuated over several years because the FDA revised its approved indications for each drug. The high incidence of adverse events highlights the importance of post-approval clinical trials and post-approval patient monitoring, to ensure that innovative personalized medicine treatments are truly improving patient outcomes. The mounting cost of EGFR inhibitor treatments highlights the need for healthcare professionals, manufacturers, and policy makers to negotiate better prices, improve insurance coverage and increase access to financial aid programs. Future efforts should focus on addressing this issue to ensure that everyone can access life-saving treatments without facing insurmountable economic burden, and to continue to enhance the safety and efficacy of TKIs.
References
[1] F. Siddiqui and A. H. Siddiqui, "Lung Cancer," *Nih.gov*, 2019. Available: View Article
[2] B. Ciupka, "SCLC vs. NSCLC: What's the Difference? | NFCR Lung Cancer Awareness," *NFCR*, Nov. 04, 2020. Available: View Article
[3] "Lung-Sparing Surgery Effective for Early-Stage Lung Cancer - NCI," *www.cancer.gov*, 2023. Available: View Article
[4] "Pneumonectomy | Types of Lung Removal Surgery | Beaumont | Beaumont Health," *www.beaumont.org*. Available: View Article
[5] G. Koulaxouzidis, G. Karagkiouzis, M. Konstantinou, I. Gkiozos, and K. Syrigos, "Sampling versus systematic full lymphatic dissection in surgical treatment of non-small cell lung cancer," *Oncology Reviews*, vol. 7, no. 1, p. 2, Jun. 2013, doi: https://doi.org/10.4081/oncol.2013.e2. View Article
[6] G. Bethune, D. Bethune, N. Ridgway, and Z. Xu, "Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update," *Journal of Thoracic Disease*, vol. 2, no. 1, pp. 48-51, 2010. Available: View Article
[7] "Gefitinib Approved for Some Lung Cancer Patients - NCI," *www.cancer.gov*, 2015. Available: View Article
[8] J.-Y. Douillard, M. Ostoros, T. Cobo, E. Ciuleanu, P. Cole, J. McCormack, J. Szczesna, L. Juhasz, R. Orlov, A. Wilson, J. Grigorescu, R. Cole, A. Nanda, and N. Korytowsky, "First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study," *British Journal of Cancer*, vol. 110, no. 1, pp. 55-62, Nov. 2013, doi: https://doi.org/10.1038/bjc.2013.721. View Article
[9] M. Fukuoka, Y. L. Wu, S. Thongprasert, P. Sunpaweravong, S. S. Leong, V. Sriuranpong, T. Y. Chao, K. Nakagawa, D. T. Chu, N. Saijo, E. L. Duffield, Y. Rukazenkov, G. Speake, H. Jiang, A. A. Armour, K. F. To, J. C. Yang, and T. S. Mok, "Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS)," *Journal of Clinical Oncology*, vol. 29, no. 21, pp. 2866-2874, Jul. 2011, doi: 10.1200/JCO.2010.33.4235. View Article
[10] J. R. Johnson, "Approval Summary for Erlotinib for Treatment of Patients with Locally Advanced or Metastatic Non-Small Cell Lung Cancer after Failure of at Least One Prior Chemotherapy Regimen," *Clinical Cancer Research*, vol. 11, no. 18, pp. 6414-6421, Sep. 2005, doi: https://doi.org/10.1158/1078-0432.ccr-05-0790. View Article
[11] "ODAC votes against erlotinib as maintenance therapy for advanced NSCLC," *www.healio.com*. Available: https://www.healio.com/news/hematology-oncology/20120331/odac-votes-against-erlotinib-as-maintenance-therapy-for-advanced-nsclc (accessed Jul. 18, 2024). View Article
[12] S. Cicènas, S. L. Geater, P. Petrov, Y. Hotko, G. Hooper, F. Xia, N. Mudie, and Y. L. Wu, "Maintenance erlotinib versus erlotinib at disease progression in patients with advanced non-small-cell lung cancer who have not progressed following platinum-based chemotherapy (IUNO study)," *Lung Cancer*, vol. 102, pp. 30-37, Dec. 2016, doi: 10.1016/j.lungcan.2016.10.007. View Article
[13] C. for D. E. and Research, "FDA broadens afatinib indication to previously untreated, metastatic NSCLC with other non-resistant EGFR mutations," *FDA*, Feb. 2019. Available: View Article
[14] M. A. S. Abourehab, A. M. Alqahtani, B. G. M. Youssif, and A. M. Gouda, "Globally Approved TKIs: Insights into Their Syntheses, Target Kinases, Biological Activities, Receptor Interactions, and Metabolism," *Molecules*, vol. 26, no. 21, p. 6677, Jan. 2021, doi: View Article
[15] "Qlik Sense," *fis.fda.gov*. Available: View Article
[16] "Centers for Medicare & Medicaid Services Data," *data.cms.gov*. Available: View Article
[17] Y. Chung, Y. Lin, M. Hung, M. Ho, & Y. Fang, "Clinical impact of epidermal growth factor receptor tyrosine kinase inhibitor associated clostridioides difficile infection among patients with lung cancer", Oncotargets and Therapy, vol. Volume 15, p. 1563-1571, 2022. View Article
[18] P. Zhao, H. Zhen, H. Zhao, L. Zhao, & B. Cao, "Efficacy and safety of adjuvant egfr-tkis for resected non-small cell lung cancer: a systematic review and meta-analysis based on randomized control trials", BMC Cancer, vol. 22, no. 1, 2022. View Article
[19]A. L. Jobe, K. T. Ahonen, V. Poplin, S. Rao, and G. R. Mohyuddin, "Afatinib-induced Interstitial Lung Disease Successfully Treated with Corticosteroids: Case Report and Review of the Literature," *Cureus*, Jun. 2018, doi: View Article
[20] F. Xing, S. Lo, S. Lau, & P. Woo, "Listeriosis in a metropolitan hospital: is targeted therapy a risk factor for infection?", Frontiers in Medicine, vol. 9, 2022. View Article
[21]R. Shenolikar, S. Liu, A. Shah, J. Tse, Y. Cao, & A. Near, "Real‐world treatment patterns of metastatic non‐small cell lung cancer patients receiving epidermal growth factor receptor tyrosine kinase inhibitors", Cancer Medicine, vol. 12, no. 1, p. 159-169, 2022. https://doi.org/10.1002/cam4.4918 View Article
[22]Nieva, J., Reckamp, K. L., Potter, D., Taylor, A., & Park, S. (2022). Retrospective analysis of real-world management of egfr-mutated advanced nsclc, after first-line egfr-tki treatment: us treatment patterns, attrition, and survival data. Drugs - Real World Outcomes, 9(3), 333-345. View Article
[23]L. Zhu, S. Gao, X. Zhao, & Y. Wang, "Identification of biomarkers, pathways, and therapeutic targets for egfr-tki resistance in nsclc", Life Science Alliance, vol. 6, no. 12, p. e202302110, 2023. View Article

