Volume 9 - Year 2022 - Pages 15-20
DOI: 10.11159/jbeb.2022.004
Analyzing Adverse Events of Mitral and Aortic Valves during the Pandemic
Elsa S Zhou1, Sujata K. Bhatia2
1Indian Hill High School
Cincinnati, Ohio, United States
elsa.sophia.zhou@gmail.com
2Harvard University
Cambridge, Massachusetts, United States
sbhatia@g.harvard.edu
Abstract - The COVID-19 pandemic forced cardiologists to adapt to unprecedented circumstances. We chose to investigate the pandemic’s effect on heart valve replacements, in particular focussing on device failure in mitral valve replacements and percutaneous aortic valve prostheses. In order to measure this effect, we examined adverse event reports of these two devices in the Food and Drug Administration (FDA)’s Manufacturer and User Facility Device Experience (MAUDE) database. We compared weekly numbers of adverse event reports during the pandemic (March 2020-March 2021) to those of the year before (March 2019-March 2020). We find that reports of deaths, injuries, and malfunctions attributed to mitral valve repair devices all showed no significant changes during the pandemic, compared to the year preceding. However, we have also found that during the pandemic, there was a 107.4% increase in deaths reported to the FDA that were attributed to percutaneous aortic valve prostheses, and a 45.1% increase in reports of malfunctions as well compared to the year preceding the pandemic. These results suggest that the pandemic may have induced an increase in transcatheter aortic valve replacements vs. surgical aortic valve replacements, leading to an increase in adverse event reports associated with percutaneous aortic valve prostheses. In contrast, transcatheter mitral valve repair is not commonly performed, and the pandemic is unlikely to have changed treatment protocols for mitral valve repair.
Keywords: Adverse events, devices, heart valves
© Copyright 2022 Authors This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2022-09-09
Date Accepted: 2022-09-20
Date Published: 2022-10-11
1. Introduction
We have previously reported that decreases of 46% and 27% for reported deaths attributed to implantable cardioverter defibrillators and reported injuries attributed to coronary drug-eluting stents, respectively, occurred during the pandemic [1]. These results led us to proffer that systemic issues such as underreporting might have been the cause of these significant decreases in adverse event reports. However, in other previous work, we found that there were significant increases in reported deaths and malfunctions of percutaneous aortic valve prostheses, suggesting that there were shifts in care patterns that heterogeneously affected different medical devices instead of one broadly applicable reason that could be identified (such as underreporting) [2]. Specifically, we proposed that a shift from surgical aortic valve replacement (sAVR) to transcatheter aortic valve replacement (TAVR) may have occurred during the pandemic due to the shorter hospital recovery period, and thus reduced risk of contracting COVID-19, resulting from the use of TAVR. However, because TAVR has not been proven to be equivalently efficacious on low-risk patient groups as compared to sAVR, it is possible that an increase in adverse events arose from this switch in care. We use this previous work as the basis for investigating mitral valve repair devices, particularly because percutaneous transcatheter interventions are not commonly performed for mitral valve repair.
2. Device Background
Mitral valve repair devices are used to treat patients with mitral regurgitation, a condition where the mitral valve does not close completely, allowing for blood to flow backward in the heart [3]. Transcatheter mitral valve repair (TMVr) is an emerging solution for mitral regurgitation that is minimally invasive whereas the traditional open-heart surgery intervention is not [4]. The leading TMVr device approved by the FDA is Abbott’s MitraClip, which has shown low rates of adverse events compared to mitral surgery [5]. Notable adverse events associated with mitral valve repair devices and the MitraClip in particular include bleeding, acute kidney failure, mitral stenosis as a result of the procedure, and structural device failure such as partial clip detachment which can result in a recurrence of mitral regurgitation [5]. A single-center retrospective analysis of 20 patients found that TMVr was associated with high short-term mortality, where 50% of patients who underwent TMVr died within 153 days [6].
Transcatheter mitral valve replacement (TMVR) has also been an intervention explored in recent years following the relative success of TAVR in working as a minimally invasive alternative to sAVR for patients with aortic stenosis. Mitral regurgitation is the most common valve disease in adults, including almost 10% of people over 75 [7]. However, TMVR is much less popular among physicians than TAVR because of the significant anatomic challenges associated with the mitral valve such as the D-shape of the mitral valve annulus [8]. Essentially, it is more difficult to anchor the mitral valve prosthesis because unlike TAVR, the valve fixation cannot be dependent only on radial forces [7]. Advances made by radiologists in computed tomography have become important to address these issues [9]. Another issue lies in the fact that many patients for whom TMVR, rather than repair, is suitable (patients at high surgical risk with functional secondary mitral regurgitation) also have tricuspid regurgitation and atrial fibrillation. Both tricuspid regurgitation and atrial fibrillation are conditions that require their own intervention, so TMVR will not sufficiently treat most patients who are most suitable for it [8]. In addition, paravalvular leak is a major issue with mitral valve replacements because of the lack of anatomic support provided by the mitral valve, as well as its positioning in relation to the circumflex coronary artery and aortic valve [8]. In contrast, paravalvular leak has been addressed with aortic valve replacement through innovation of devices that mitigate the occurrence of the adverse event [8]. Adverse events associated with TMVR include left ventricle outflow tract obstruction, thrombosis, and stroke [10]. Left ventricle outflow tract obstruction has a nearly 62% in-hospital mortality [7].
Aortic valve replacement is a procedure used to treat aortic stenosis, which is a common vascular disease in developed countries [11]. Transcatheter aortic valve replacement in particular has become a popular alternative to sAVR in recent years, in part because it has been associated with a 50% reduction in hospital recovery periods for patients. Specifically, annual TAVR volumes have been increasing since 2012, even exceeding sAVR volume in 2019 [12]. However, TAVR has also been associated with significantly higher rates of paravalvular leak and major vascular complications when compared to sAVR [11]. Still, several clinical trials have demonstrated the noninferiority of TAVR compared to sAVR, and the PARTNER-1 trial has shown the adequate hemodynamic profile of TAVR at 5-year follow-up [13]. In a study with 358 patients, TAVR was shown to be more beneficial than standard treatments such as balloon aortic valvuloplasty for treating inoperable aortic stenosis [14].
3. Methods
We used adverse event data from the Food and Drug Administration (FDA)’s Manufacturer and User Facility Device Experience (MAUDE) database, which compiles adverse event reports from manufacturers, distributors, physicians, and voluntary reporters such as patients themselves [15]. Various filters can be applied to this database, and we filtered by device and adverse event type. The device we examined was labelled ‘Mitral Valve Repair Devices’ and we examined the adverse event types ‘Death’, ‘Injury’, and ‘Malfunction’. We chose to analyse data from every week over the course of three years, beginning with the first year of the pandemic and working backward. The World Health Organization’s classification of COVID-19 as a pandemic in march of 2020 prompted us to consider the first year of the pandemic as March 2020-March 2021 [16]. The year preceding was thus March 2019-March 2020, and two years preceding was March 2018-March 2019. We performed paired t-tests to analyse the differences in adverse event reports per week when comparing trends between two years.
4. Results
We report that mitral valve repair devices showed somewhat significant increases in reports of all three adverse event types during the year preceding the pandemic, compared to two years preceding the pandemic, but that all three adverse event types did not exhibit significant changes in adverse event reports per week during the pandemic.
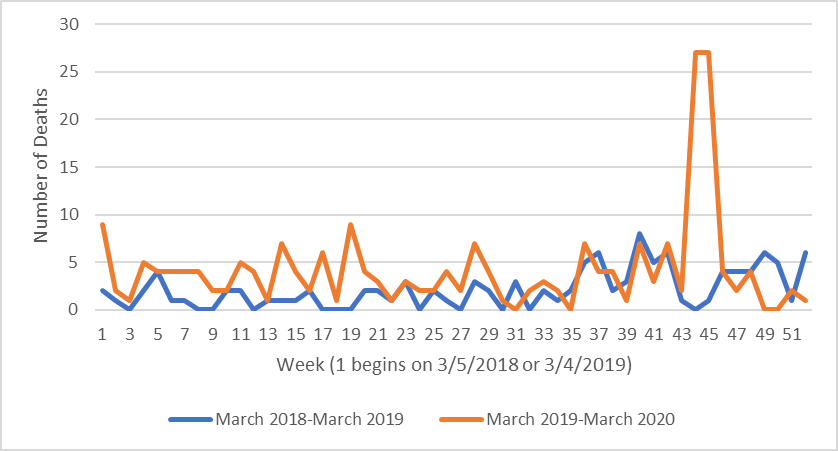
For reported deaths caused by mitral valve repair devices, there was a 96.4% increase during the year preceding the pandemic (March 2019-March 2020) when compared to two years preceding the pandemic (March 2018-March 2019), translating to 2.1 more reports per week (P-value = .01)(Figure 1). However, this result is only moderately significant, especially in comparison to the results observed in our previous works, where the P-value for the decreases in death reports for ICDs was < .0001, for example. When comparing adverse event data during the pandemic to the year prior, we found no significant change in rates of deaths reported that were caused by mitral valve repair devices (Mean = .13 reports, P-value = .87).
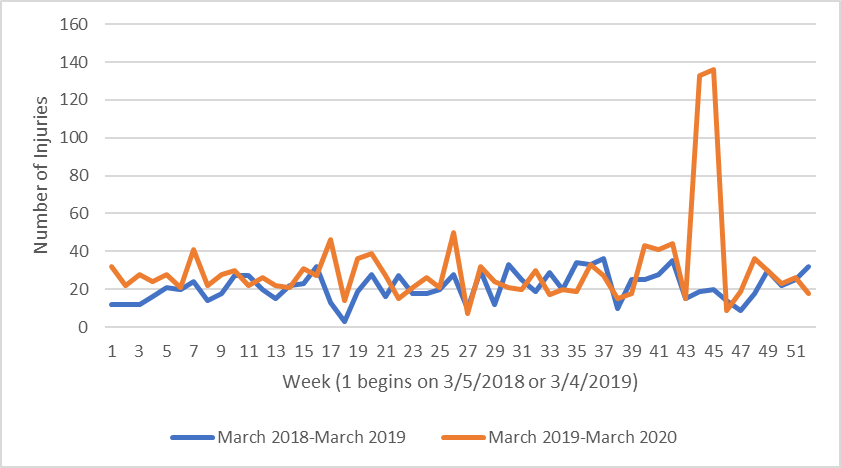
For reported injuries attributed to mitral valve repair devices, we found a similar situation. There were 8.9 more reports per week, or a 41.6% increase during the year prior to the pandemic when compared to two years prior (P-value = .0090)(Figure 2). Again, however, the result is only moderately significant, and we found no significant change in adverse event reports per week during the pandemic (Mean = -2.9 reports, P-value = .37).
There is noticeably a spike in both reported deaths and injuries attributed to mitral valve repair devices from Week 43 to Week 46 starting from March 4, 2019, translating to December 23, 2019 to January 19, 2020. In both deaths and injuries, all reports were of Abbott’s MitraClip device.
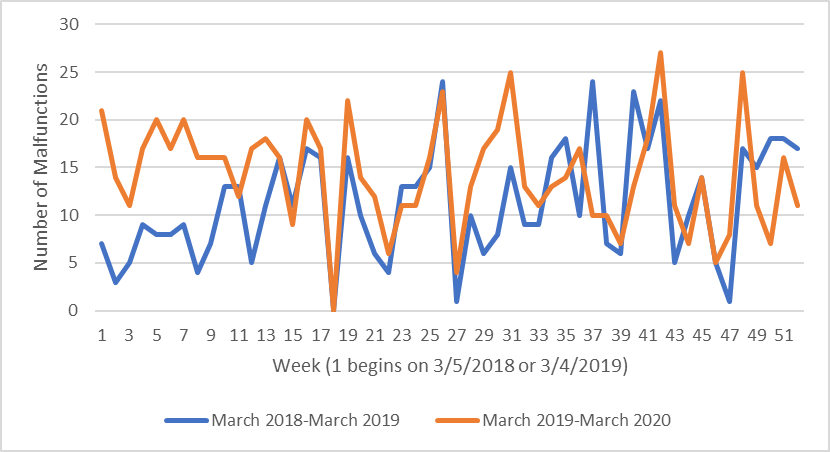
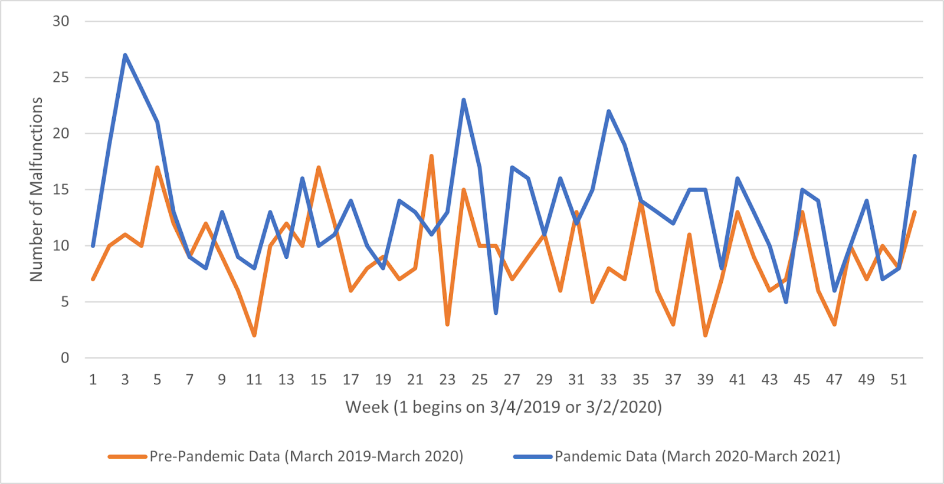
As for malfunctions reported to the FDA that were attributed to mitral valve repair devices, there were 3.0 more reports per week during the year preceding the pandemic compared to two years prior, making for a 26.4% increase (P-value = .0012)(Figure 3). This result was more significant than the results from mitral valve repair device-attributed injuries and deaths. Similar to the injuries and death adverse event report trends for mitral valve repair devices, we found no significant change in reported malfunction trends during the pandemic (Mean = 1.4 reports, P-value = .21).
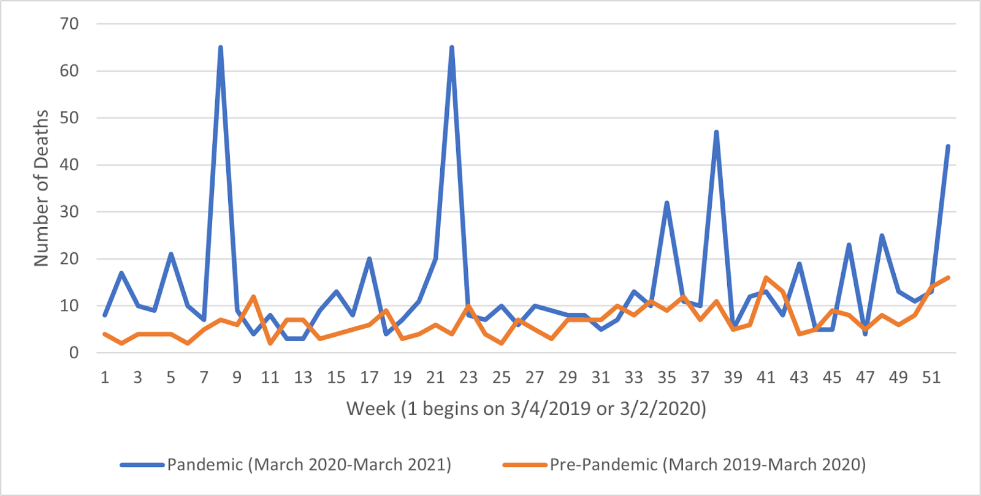
For reference, also included are the figures and findings for percutaneous aortic valve prostheses [2]. We found that during the pandemic, reported deaths attributed to percutaneous aortic valve prostheses rose by 107.4% (P-value < .0005)(Figure 4).
We also found that during the pandemic, percutaneous aortic valve prosthesis-attributed injuries reported to MAUDE rose by 45.1% (P-value < .0001)(Figure 5).
5. Figures
6. Discussion
The individual surgeon volumes have been shown to impact operative mortality rate as well as rates of mitral repair over replacement. More experienced surgeons were able to more successfully treat more patients, indicative of a learning curve [17]. A study with 313 surgeons involving treatment of degenerative mitral valve disease showed that with every 10-case increment in total annual surgeon volume, there was an associated 13% increase in repair rates, where repair rates were calculated by dividing the number of repairs a surgeon performed by the total number of procedures performed to address degenerative mitral valve disease [18].
We previously suggested that a switch from sAVR to TAVR for low-risk patients might have led to the increases in reported injuries and malfunctions attributed to percutaneous aortic valve prostheses. This is because TAVR holds a logistical advantage compared to sAVR: it is minimally invasive, therefore taking up less hospital beds as well as reducing likelihood of patient exposure to COVID-19 [19]. However, for patients with low surgical risk, TAVR has not been proven to be as efficacious as sAVR [20]. Therefore, there is the possibility that the use of TAVR in low-risk patients during the pandemic due to its advantages for minimizing spread of infectious disease could have resulted in a higher rate of malfunctions and injuries. TAVR in low-risk patients may be associated with higher rates of permanent pacemaker implantation, which has been associated with almost a doubling in risk of heart failure admission [21]. Certainly, more research must be conducted to determine if this suggestion is truly a causal factor in the observed increase in adverse event reports for percutaneous aortic valve prostheses. Still, given that a shift to TAVR is a potential explanation, it is worthwhile examining the similarities and differences between TAVR and mitral valve interventions such as TMVr and TMVR.
The most significant differences are the difficulty of a successful TMVR procedure, which involve anatomic challenges such as anchoring of the prosthesis and whose post-procedural risks of paravalvular leak and cardiovascular comorbidities may cause complications. Other differences include demographic differences. Patients undergoing TMVR are on average around 10 years younger than those undergoing TAVR [13]. A shift to TMVR during the pandemic, from either TMVr or surgical mitral valve interventions, is unlikely because of the difficulties mentioned earlier in successfully replacing a mitral valve. Also, TMVr may be preferred over TMVR in some cases. Especially for degenerative mitral valve disease, which constitutes 60-70% of surgical mitral regurgitation, repair is strongly preferred over replacement [22, 23]. Thus, the analyses that show no significant changes in reports of death, injury, or malfunction attributed to mitral valve repair devices are expected.
Even with a possibility of pandemic-related underreporting of adverse events, there is a dramatic increase in reported adverse events for percutaneous aortic valve prostheses. This increase is likely caused by a shift from sAVR to TAVR. With the analyses of mitral valve repair devices, it is unlikely that transcatheter mitral valve interventions experienced the same shift in care, especially when considering the debate between mitral valve repair (TMVr) and mitral valve replacement (TMVR) for a variety of conditions. This can explain the analyses which show no significant change in adverse events over the years investigated.
The pandemic appears to have exacerbated the shift from sAVR to TAVR, but certainly the shift was already occurring before the pandemic. This suggests that the pandemic acted more as a compounding factor rather than the sole cause for the changes in adverse events displayed in the data, and that potential future pandemics or infections may continue to be a factor in the shift from sAVR to TAVR but will not ultimately be the cause for the shift.
4. Conclusion
We conclude that adverse event data collected by the FDA reveals that the weekly number of reports of mitral valve repair device failure (malfunction, death, and injury) increased before the pandemic, but that no significant change occurred during the first year of the pandemic. Conversely, we previously found that the weekly number of reports of aortic valve prostheses deaths and injuries increased during the first year of the pandemic. This may reflect the different applications of the two devices due to a variety of factors that distinguish the devices, and even the patients undergoing procedures for the implantation of the devices, from each other. These data may help us better understand the effects of the pandemic on healthcare and cardiology as a whole by indicating which devices are showing significantly higher numbers of adverse event reports, which devices’ failure rates seem relatively unaffected by the pandemic, and which devices reveal pauses in previous years’ trends of increasing or decreasing reported rates of failure. These insights will then allow us to identify and address important gaps in patient care. Further research should aim to pinpoint the cause of the trends shown in the adverse event report data of this paper and continue to analyze other devices and their reported failures.
References
[1] E.S. Zhou and S.K. Bhatia, “Decrease in Reported Rates of Cardiovascular Device-Related Adverse Events During the Coronavirus Disease 2019 Pandemic,” Am J Cardiol., In Press.
[2] E.S. Zhou and S.K. Bhatia, “Divergent Effects of COVID-19 Pandemic on Reported Adverse Events for Percutaneous Aortic Valve Prostheses and Non–Allograft Tissue Valves,” Am J Cardiol., In Press.
[3] Mayo Clinic (2022, Jan 4). Mitral valve regurgitation [Online]. Available: View Article
[4] Stanford Healthcare (2022, Jan 4). Transcatheter Mitral Valve Repair (TMVR) [Online]. Available: View Article
[5] K. Schnitzler, M. Hell, M. Geyer, F. Kreidel, T. Munzel, and R.S. von Bardeleben, “Complications Following MitraClip Implantation,” Curr Cardiol Rep., vol. 23, no. 9, pp. 131, 2021. View Article
[6] C.P. Kovach, S. Bell, A. Kataruka, M. Reisman, and C. Don, “Outcomes of urgent/emergent transcatheter mitral valve repair (MitraClip): A single center experience,” Catheter Cardiovasc Interv., vol. 97, no. 3, pp. 402-410, 2021. View Article
[7] E. Rawish, T. Schmidt, I. Eitel, and C. Frerker, “Current Status of Catheter-based Mitral Valve Replacement,” Curr Cardiol Rep., vol. 23, no. 8, pp. 95, 2021. View Article
[8] M.C. Wyler von Ballmoos, A. Kalra, and M.J. Reardon, “Complexities of transcatheter mitral valve replacement (TMVR) and why it is not transcatheter aortic valve replacement (TAVR),” Ann Cardiothorac Surg., vol. 7, no. 6, pp. 724-730, 2018. View Article
[9] R. Wilson, C. McNabney, J.R. Weir-McCall, S. Sellers, P. Blanke, and J.A. Leipsic, “Transcatheter Aortic and Mitral Valve Replacements,” Radiol Clin North Am., vol. 57, no. 1, pp. 165-178, 2019. View Article
[10] P. Overtchouk, N. Piazza, J.F. Granada, and T. Modine, “Predictors of adverse outcomes after transcatheter mitral valve replacement,” Expert Rev Cardiovasc Ther., vol. 17, no. 8, pp. 625-632, 2019. View Article
[11] J. Spears, Y. Al-Saiegh, D. Goldberg, S. Manthey, and S. Goldberg, “TAVR: A Review of Current Practices and Considerations in Low-Risk Patients,” J Interv Cardiol., In Press.
[12] J.D. Carroll, M.J. Mack, S. Vemulapalli, H.C. Herrmann, T.G. Gleason, G. Hanzel, G.M. Deeb, V.H. Thourani, D.J. Cohen, A.J. Kirtane, S. Fitzgerald, J. Michaels, C. Krohn, F.A. Masoudi, R.G. Brindis, and J.E. Bavaria, “STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement,” J Am Coll Cardiol, vol. 76, no. 21, pp. 2494-2516, 2020. View Article
[13] L. Testa, A.P. Rubbio, M. Casenghi, G. Pero, A. Latib, and F. Bedogni, “Transcatheter Mitral Valve Replacement in the Transcatheter Aortic Valve Replacement Era,” J Am Heart Assoc., In Press.
[14] S.R. Kapadia, M.B. Leon, R.R. Makkar, E.M. Tuzcu, L.G. Svensson, S. Kodali, J.G. Webb, M.J. Mack, P.S. Douglas, V.H. Thourani, V.C. Babaliaros, H.C. Herrmann, W.Y. Szeto, A.D. Pichard, M.R. Williams, G.P. Fontana, D.C. Miller, W.N. Anderson, J.J. Akin, M.J. Davidson, C.R. Smith, and PARTNER Trial investigators, “5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial,” Lancet, vol. 385, no. 9986, pp. 2485-2491, 2015. View Article
[15] Food and Drug Administration (2022, Jan 4). MAUDE – Manufacturer and User Facility Device Experience [Online]. Available: View Article
[16] D. Cucinotta, M. Vanelli, “WHO Declares COVID-19 a Pandemic,” Acta Biomed., vol. 91, no. 1, pp. 157-160, 2020.
[17] J.H. Kang, S.J. Bozso, R. El-Andari, C. Adams, and J. Nagendran, “Transcatheter mitral valve repair and replacement: the next frontier of transcatheter valve intervention,” Curr Opin Cardiol., vol. 36, no. 2, pp. 163-171, 2021. View Article
[18] J. Chikwe, N. Toyoda, A.C. Anyanwu, S. Itagaki, N.N. Egorova, P. Boateng, A. El-Eshmawi, and D.H. Adams, “Relation of Mitral Valve Surgery Volume to Repair Rate, Durability, and Survival,” J Am Coll Cardiol., vol. 69, no. 19, pp. 2397-2406, 2017. View Article
[19] C. Basman, C.A. Kliger, L. Pirelli, and S.J. Scheinerman, “Management of elective aortic valve replacement over the long term in the era of COVID-19,” Eur J Cardiothorac Surg., vol. 57, no. 6, pp. 1029-1031, 2020. View Article
[20] B. Khialani and P. MacCarthy, “Transcatheter management of severe aortic stenosis during the COVID-19 pandemic,” Heart., vol. 106, no. 15, pp. 1183-1190, 2020. View Article
[21] K.V. Patel, W. Omar, P.E. Gonzalez, M.E. Jessen, L. Huffman, D.J. Kumbhani, and A.A. Bavry, “Expansion of TAVR into Low-Risk Patients and Who to Consider for SAVR,” Cardiol Ther., vol. 9, no. 2, pp. 377-394, 2020. View Article
[22] S.L. Mick, S. Keshavamurthy, and A.M. Gillinov, “Mitral valve repair versus replacement,” Ann Cardiothorac Surg., vol. 4, no. 3, pp. 230-237, 2015.
[23] R.A. Chemtob, P. Wierup, S. Mick, M. Gillinov, "Choosing the 'Best' surgical techniques for mitral valve repair: Lessons from the literature," _J Card Surg._, vol. 34, no. 8, pp. 717-727, 2019.

