Volume 9 - Year 2022 - Pages 10-14
DOI: 10.11159/jbeb.2022.003
Malfunctions & Injuries Attributed to CT Scanners Before and After the Pandemic: A Retrospective Analysis
Derry Li1, Sujata K. Bhatia2
1Methacton High School
1001 Kriebel Mill Road, Eagleville, Pennsylvania, United States 19403
derryxli@gmail.com;
2Harvard University, Department of Biomedical Engineering
51 Brattle Street, Cambridge, Massachusetts, United States 02138
sbhatia@g.harvard.edu
Abstract - Given the stresses placed on healthcare during the COVID-19 pandemic, and the critical role of computed tomography (CT) scanners in diagnosing cancers and other disorders, this project is designed to investigate the impact of the COVID-19 pandemic on reported malfunctions, injuries (and deaths) attributed to CT scans. Data were extracted from the Food and Drug Administration (FDA) Manufacturer and User Facility Device Experience (MAUDE) database. Yearly numbers of adverse event (AE) reports attributed to CT scanners including malfunctions, injuries, and deaths were recorded for the last 10 years (2012 to 2021). Monthly numbers of reports were also recorded for the 12 months immediately preceding the pandemic (2019/03 to 2020/03), as well as all the months after WHO declared COVID-19 a global pandemic (since 2020/03). It was found that the reported rates of injuries and malfunctions for CT scanners increased during the COVID-19 pandemic. The analysis also revealed unusual trends such as spikes in the malfunction rates from 2015 to 2018 compared to the preceding years, as well as in injuries and deaths. Manufacturers most responsible for these AE spikes included Philips, Superdimension, GE Healthcare, Siemens, etc. The FDA Recall Database was further mined, and similar trends were identified in the yearly recalls over 2015- 2018, which correlated well with the malfunction rates (less apparent for injuries and deaths). While this project was originally centered around adverse pandemic-related effects on CT scanners, the important pre-pandemic findings warrant further research. These results might help prevent future AEs caused not only by CT scans but also by other medical devices.
Keywords: Computed tomography, COVID-19 Pandemic, Adverse Event, Malfunctions, Injuries
© Copyright 2022 Authors This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2022-08-28
Date Accepted: 2022-09-11
Date Published: 2022-09-15
1. Introduction
The COVID-19 pandemic, one of the most paramount problems in the modern world, has fundamentally changed people’s lives in the last few years. While the increased workloads and restrictions are placed to effectively prevent the spread of the COVID-19 virus, they also limit the ability of technicians and medical staff to perform their jobs and maintain machinery up to specifications. Reported adverse effects resulting from the national crisis include mental stress, heavier workloads, heavy health restrictions, etc. [1].
Computed Tomography (CT) technology, one of the widely used medical imaging devices, scans the body and computerizes the cross-sectional images into three-dimensional images that are used to identify bones, muscles, and other soft tissues [2]-[4]. These images allow professionals to identify internal structures and make diagnoses for injuries and diseases, especially tumors and internal bleeding. CT scanners have also been used to provide image assistance to surgeons to enhance the precision of surgery procedures and avoid unnecessary damages [5]. However, CT scanners, like other imaging devices, can have malfunctions with inappropriate use and when used under stress, like during the COVID-19 pandemic. The prolonged stresses on hospitals and medical staff may contribute to a lack of maintenance for CT scanners and CT assessment.
This research analyzes the reported number of events for malfunctions, injuries, deaths, and recalls attributed to CT scanners during the pandemic versus that in pre-pandemic. The trends observed are important to identify the underlying contributing factors to these adverse events and to assess the impact of the pandemic. This is critical because if unidentified, such factors could lead to more fatal injuries and continued detriment to patients who need CT assessment. It may also help us to prevent future adverse events caused by CT scanners and other medical devices in times of limited medical resources.
Two device-associated public searchable online databases are utilized in this work, the Manufacturer and User Facility Device Experience (MAUDE) database [6] and the Medical Device Recall database [7]. Device events are reported to the FDA based on functionality problems or severity of the case at or around the time of use of the equipment and archived in the MAUDE database. The recall database provides data in a similar format to MAUDE; it lists recall events from manufacturers or requests for recall back to the manufacturer for repairs regarding machinery problems that could pose serious injury or are detrimental to the healthcare process.
2. Methods
The FDA MAUDE database was searched using the search terms ‘System, X-Ray, Tomography, Computed’ for the product class and ‘Malfunction’, ‘Injures’, or ‘Death’ for the event types. The FDA recall database was also searched using the search terms “JAK” for the product code. The searches, therefore, returned all instances of the corresponding events reported for CT scanners where the device manufacturer was recorded for each case. These data were used to estimate the number of reported monthly and yearly events in the last 10 years (2012 to 2021), as well as reports per manufacturer. Specifically, results from the periods 12 months before when WHO declared Covid-19 a global pandemic (March 2020) [8] and periods afterward (up to December 2021) were explored and compared (using a student t-test).
3. Results
A total of 1664, 913, and 192 cases of malfunction, injuries, and deaths, respectively, attributed to CT Scanners were found from January 2012 to December 2021 via the search of the FDA MAUDE database. A total of 544 recall events attributed to CT Scanners were identified in the same period via the search of the FDA recall database.
Analysis of the malfunction event data revealed an increase in the number of monthly reported malfunction events before the COVID-19 Pandemic (March 2019 to February 2020) compared to those after (March 2020 to December 2021) (Figure 1). This was more apparent in the second surge of COVID cases (December 2020 to May 2021, p < 0.05), likely due to stresses placed on the healthcare system by the continued pandemic and the reintroduction of patients to a normal volume of medical diagnostic procedures. No trends were evident for the adverse events of injury and death (data not shown).
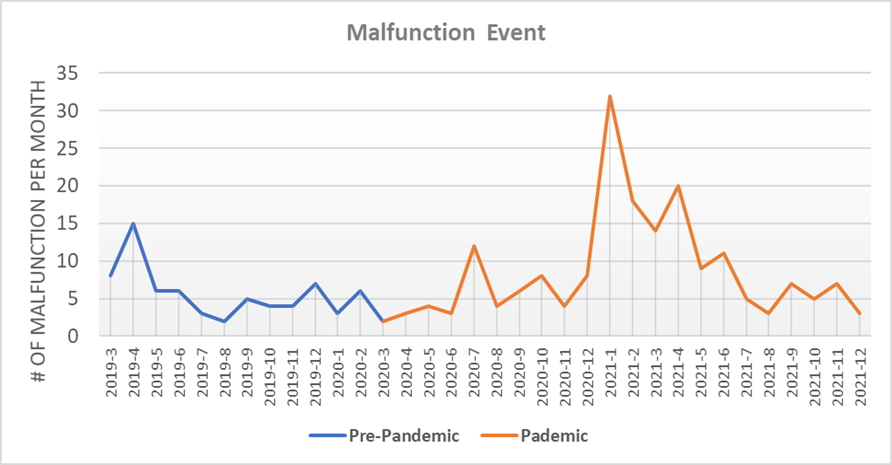
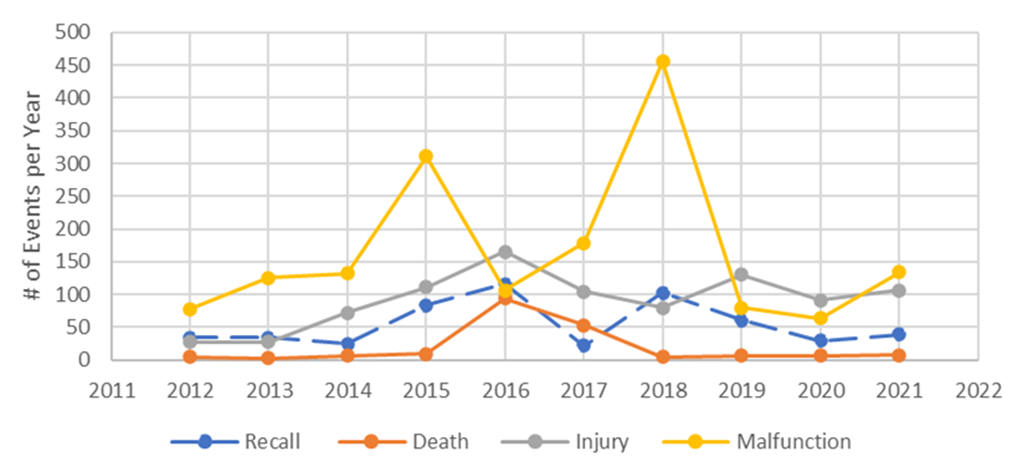
Figure 2 described the number of yearly reported events associated with CT scanners from 2012 to 2021, including malfunctions, injuries, and deaths. Unusual spikes were seen in the number of malfunction events reported from 2015 to 2018, compared to the preceding years. Similar trends appeared in the reported number of injuries and deaths.
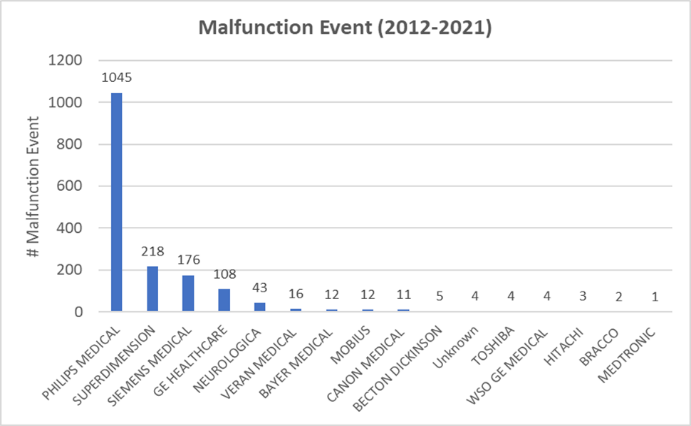
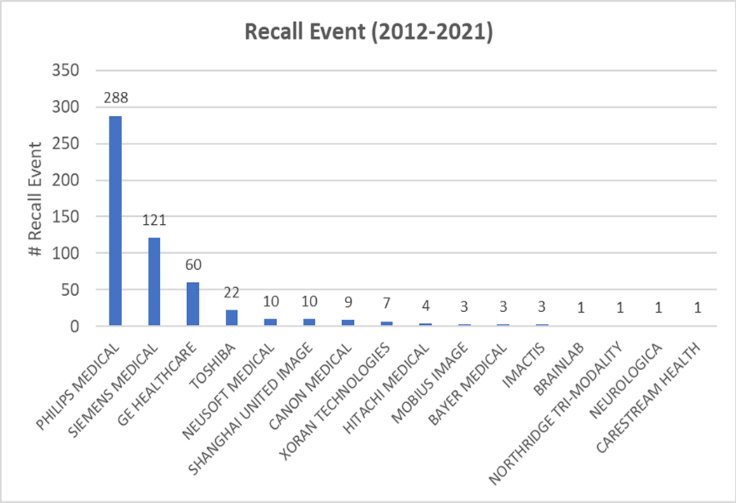
The number of yearly recalls were also shown in the same plot (Figure 2), where its trend appeared to follow that of malfunction, indicating a potential correlation between malfunction events and recalls. Further sub-group analysis of reported events by manufacturers indicated that this could be partially related to the corresponding manufacturers. One company, Philips Medical, seems to be associated with the greatest total number of malfunction and recall events from 2012 to 2021 (Figure 3 & Figure 4). Other major companies involved were Superdimension, GE healthcare, and Siemens. These trends were more obvious during 2015 and 2018 based on the subsequent sub-group analysis by year and by the manufacturer (data not shown).
4. Discussion
This work suggests that reported rates of injuries and malfunctions for CT scanners increased during the COVID-19 pandemic, supporting the hypothesis that the pandemic had an adverse effect on CT scanner use. Other medical imaging devices likely experienced similar effects [9], supporting the notion that the pandemic placed serious stress on the healthcare system. The increased number of events in the second but not first surge of COVID-19 was not unexpected. Due to health restrictions placed on the public and general fear of infection, clinical visits decreased dramatically during the first wave of the pandemic; vaccinations were introduced later in 2020 and early 2021. As visit rates returned to the status quo [10], cumulative CT scanner usage combined with suboptimal device performance may have led to an increase in malfunctions.
There are other possible explanations for the observed results. For instance, there may have been a quality issue for a specific medical device which coincided with the pandemic; however, a search of the FDA Device Recall Database reveals that there were no recalls of CT scanners during the pandemic, making this explanation less likely. Another possible explanation for the observed results is that patients may have simply been sicker during the COVID-19 pandemic, due to undetected infections, and this could have led to increased adverse event reports. The best way to address this question will be to examine individual adverse event narratives, as well as the impact of the COVID-19 pandemic on adverse event reporting for other imaging modalities, and this is an avenue for future research. Another possibility is that any infectious pandemic could increase adverse event rates for imaging devices; this question can be addressed by examining adverse event rates for CT scanners during past pandemics, such as the H1N1 influenza pandemic. Again, this will be an avenue for future research.
This analysis also revealed unusual spikes in the malfunction rates from 2015 to 2018 compared to the preceding years (also seen in injuries and deaths). The FDA Recall Database was mined, and similar trends were identified in the yearly recalls from 2015 to 2018. Manufacturers most responsible for these spikes included Philips, Superdimension, GE healthcare, and Siemens. These are major companies in the medical imaging market that have similar market shares [11]. As Philips was associated with the greatest reported number of malfunctions and recalls based on the current search, it may have a high chance of abnormally large volumes of malfunctioning CT scanners compared to other manufacturers based on market shares. While it is true that the manufacturers with the greatest volume of cases and reports may also be the largest distributors of the device, the absolute volume and total sum of events cannot be overlooked, as they indicate a ‘real-world’ estimate of how many people are being affected, regardless of how many ‘malfunction’ devices exist in circulation in proportion to total available devices.
One limitation of this research is that the FDA MAUDE database relies on both mandatory and voluntary reports of adverse events. Mandatory reporters include device manufacturers, importers, and user facilities. Voluntary reporters include healthcare professionals, patients, and consumers. Under-reporting, mis-reporting, and duplicate reports are all possible in the FDA MAUDE database. However, analysis of FDA MAUDE report frequencies can uncover trends in device adverse events. Again, future research should examine individual event narratives to validate the observed increases in adverse events for CT scanners during the pandemic.
4. Conclusion
In summary, this study demonstrates that reported rates of injuries and malfunctions for CT scanners increased during the COVID-19 pandemic, supporting the hypothesis that the pandemic had an adverse effect on CT scanner use. This study also reveals unexpected spikes in CT scanner malfunction rates from 2015 to 2018 compared to the preceding years (also seen in injuries and deaths). Future research should examine individual event narratives to discover the cause of increased adverse events for CT scanners during the pandemic. Moreover, future research should examine adverse event rates for other imaging modalities during the COVID-19 pandemic. Finally, future research should examine the impacts of past pandemics, such as the H1N1 influenza pandemic, on adverse event reporting for CT scanners and other imaging devices.
References
[1] Couarraze S, Delamarre L, Marhar F, Quach B, Jiao J, Dorlhiac RA, Saadaoui F, Liu AS, Dubuis B, Antunes S, Andant N, Pereira B, Ugbolue UC, Baker JS, The COVISTRESS network, Clinchamps M, Dutheil F, “The major worldwide stress of healthcare professionals during the first wave of the COVID-19 pandemic - the international COVISTRESS survey,” in PLOS One, Oct. 2021 [Online] Available: View Article
[2] “Computed Tomography (CT)” From NIH. [Online]. Available: View Article - (accessed February, 2022).
[3]“Computed Tomography (CT) Scan” From Mayo Clinic. [Online]. Available: View Article - (accessed February, 2022).
[4] “Computed Tomography (CT) Scan” From John Hopkins Medicine. [Online]. Available from: View Article - (accessed February, 2022)
[5]“Computed Tomography (CT) Image-Guided Surgery” From UPMC. [Online]. Available: View Article - (accessed January, 2022).
[6] Manufacturer and User Facility Device Experience, U.S. Food and Drug Administration, 1991. [Online]. Available: View Article
[7] Medical Device Recalls, U.S. Food and Drug Administration, 2002. [Online]. Available: View Article
[8] Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157-160. Published 2020 Mar 19. doi:10.23750/abm.v91i1.9397
[9] Tan BS, Dunnick NR, Gangi A, Goergen S, Jin ZY, Neri E, Nomura CS, Pitcher RD, Yee J, Mahmood U, “RSNA International Trends: A Global Perspective on the COVID-19 Pandemic and Radiology in Late 2020,” in Radiology, Dec. 2020. [Online]. Available: View Article
[10] Cobo-Calvo A, Zabalza A, Río J, Arrambide G, Otero-Romero S, Tagliani P, Cárdenas-Robledo S, Castillo M, Espejo C, Rodriguez M, Carbonell P, Rodríguez, Midaglia L, Vidal-Jordana Á, Tur C, Galan I, Castillo J, Comabella M, Nos C, Auger C, Tintoré M, Rovira Á, Montalban X, Sastre-Garriga J, Impact of COVID-19 pandemic on frequency of clinical visits, performance of MRI studies, and therapeutic choices in a multiple sclerosis referral centre. J Neurol. 2022;269(4):1764-1772. doi:10.1007/s00415-021-10958-z View Article
[11] Vianny G. “Global top 10 companies based on diagnostic imaging market share in 2017 and 2024.” From Statista. [Online]. View Article - (accessed February 2021).

