Volume 9 - Year 2022 - Pages 06-09
DOI: 10.11159/jbeb.2022.002
Unexpected Decline in Insulin Adverse Events Signals a Possible Decline in Insulin Usage
Alek Karagozyan1, Sujata K. Bhatia2
1Belmont High School
221 Concord Ave, Belmont, MA 02478, United States
alekkaragozyan2023@gmail.com
2Harvard University
51 Brattle St, Cambridge, MA 02138, United States
sbhatia@g.harvard.edu
Abstract - The Centers for Disease Control and Prevention (CDC) reports that 23.4 million people in the US were diagnosed with diabetes in 2015. Further, the National Diabetes Statistics Report for 2020 states that cases of diabetes have risen to 34.2 million, with 1.5 million new cases annually. Another alarming trend is the rise in insulin costs, making it difficult for many Americans to afford treatment. The American Journal of Managed Care indicates that the price for a vial of insulin rocketed from $21 in 1996 to $275 in 2019, which appears to be a crisis in the making. In relation to these trends, this research tracks adverse event reports for insulin and insulin pumps. The FDA Adverse Event Reporting System (FAERS) database reveals that adverse event reports for insulin from 2018 to 2021 show a steady decrease in the number of incidents. Similarly, the FDA Manufacturer and User Facility Device Experience (MAUDE) database indicates a decrease in incident reports for common insulin pumps within the same time period. There are two possible explanations for this counterintuitive trend. Since it is highly unlikely based on the data that there are fewer diabetics using insulin, there could be a decrease of usage based on the economics of acquiring insulin and related devices. The other possible explanation is that the advent and rapid adoption of continuous glucose monitoring devices has allowed patients to better regulate their insulin intake, leading to lower levels of usage. Further studies will be needed to determine root causes of these trends.
Keywords: diabetes, insulin, continuous glucose monitoring, insulin pump, adverse events
© Copyright 2022 Authors This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2022-08-23
Date Accepted: 2022-08-29
Date Published: 2022-09-01
1. Introduction
Diabetes mellitus (simply known as diabetes) is characterized by insulin deficiency or insulin resistance, causing an individual’s blood sugar to rise to an unhealthily high level for an extended period of time [11]. If not treated, diabetes dramatically shortens a person’s life span through long-term cardiovascular disease, stroke, and other serious complications. The main cause of diabetes is that either the pancreas fails to produce sufficient levels of the hormone insulin, or the insulin is not processed properly by the body. Diabetes is commonly managed through insulin injections and oral medications [12].
The Centers for Disease Control (CDC) reports that 23.4 million people in the US were diagnosed with diabetes in 2015 [1]. Further, the National Diabetes Statistics Report for 2020 states that cases of diabetes have risen to 34.2 million [2], with 1.5 million new cases annually. Another alarming trend is the rise in insulin costs, making it difficult for many Americans to afford treatment. The American Journal of Managed Care indicates that the price for a vial of insulin rocketed 1309% from $21 in 1996 to $275 in 2019 [3], which appears to be a crisis in the making. For comparison, over the time period from 1996 to 2019, overall inflation was 63%, meaning that the price increase for insulin was more than 20 times the rate of inflation.
Many patients suffering from diabetes need to perform frequent pricking of their fingers to measure sugar levels in order to decide whether to inject themselves with insulin. This traditional method is not only uncomfortable but is also infrequent as patients tend to only want to prick their fingers a few times a day, so the requirement for finger pricking may reduce patient compliance with glucose monitoring [13]. The advent of continuous glucose monitoring devices has dramatically changed how sugar levels are measured and maintained as patients are able to receive alerts and therefore take immediate action [14]. This relatively new technology not only is more convenient but also provides more opportunities to correct the patients’ sugar levels throughout their day. From the first FDA approval in 1999 with zero sales [4], glucose monitor sales have expanded rapidly to over $3 billion in 2020 [5].
With dramatic increases in diabetic patients, we would expect insulin use to also increase and reflect this growth. Using data from the FDA Adverse Event Reporting System (FAERS) database, we uncovered a surprising trend which appears to suggest a possible decline in insulin use. The FAERS database includes millions of adverse event reports from the pharmaceutical industry, healthcare providers and consumers, all of which are publicly accessible. The adverse event reports are dated and organized by specific pharmaceutical types and, as such, can be used as a measure of usage for certain products over time.
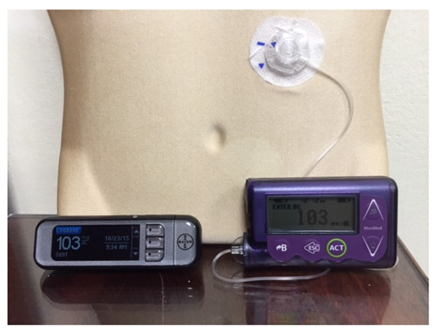
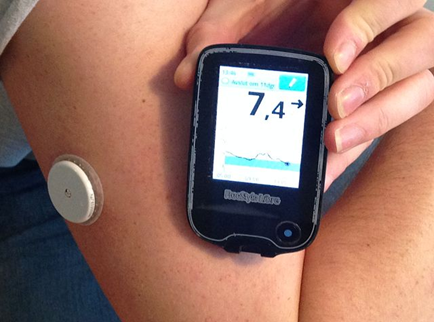
Using data from the FDA Manufacturer and User Facility Device Experience (MAUDE) database, we also examined trends in insulin pump and glucose monitor use. An insulin pump is a small computerized device that delivers insulin through a thin tube which goes under the patient’s skin (Figure 1). A continuous glucose monitor is a small sensor inserted under the patient’s skin to periodically measure glucose levels every few minutes (Figure 2).
2. Methods
We queried the FAERS database specifically for adverse events related to 55 types of insulin products. On the FAERS Public Dashboard, we used the search term “Insulin”, which yielded the list of appropriate products. Selecting each product from the list generated a table displaying the number of events for that product per year. In order to look beyond the pandemic’s potential impact on the trend, we selected only the data points from 2016 through 2021. We then summed the 55 products’ adverse events per year to compute a grand total of insulin adverse events per year, and did so for all years of interest. In addition to FAERS, we also queried the MAUDE database for usage patterns of 7 categories of insulin pumps and 4 categories of continuous glucose monitors. We used openFDA API queries to extract the necessary data. For instance, we executed a specific query for intervals from January 1 to December 31 for each year of interest in order to obtain a list of product categories, each of which contained the number of adverse events for all products in that category. We only considered categories that contained the phrases “insulin pump” and “continuous glucose monitor” for our data collection. Similarly to our insulin adverse event queries, we then added each category’s adverse event reports into a grand total for each year from 2016 through 2021.
3. Results and Discussion
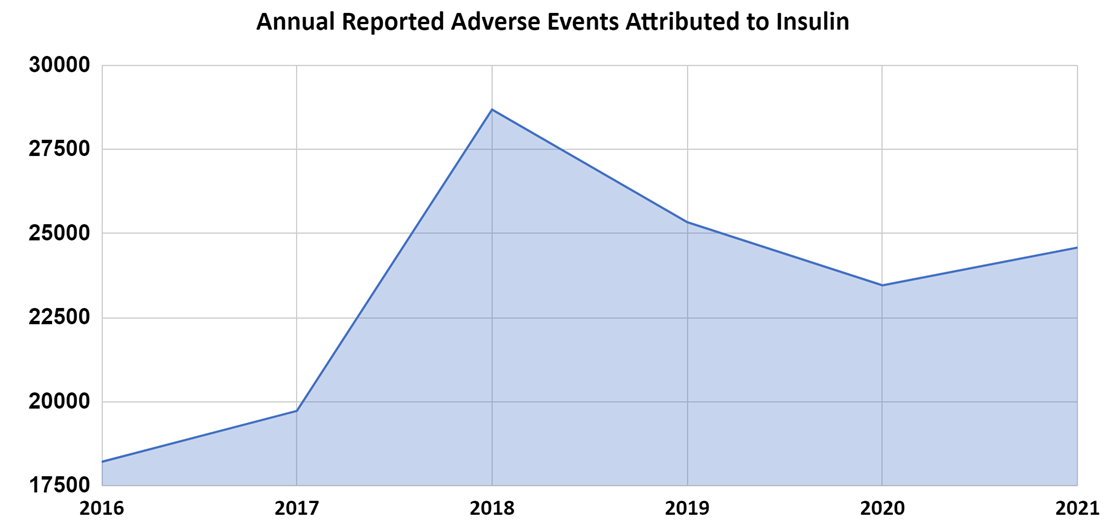
The FAERS data indicate that adverse events increased 8.3% from 2016 to 2017, 45.4% from 2017 to 2018 but surprisingly started to decrease 11.7% from 2018 to 2019 (pre-pandemic) and continued to decline 7.4% from 2019 to 2020. We also notice that 2021 shows a small 4.8% increase, which may be attributed to a post-pandemic resurgence in medical visits while the overall trend from 2018 to 2021 remains downward (Figure 3).
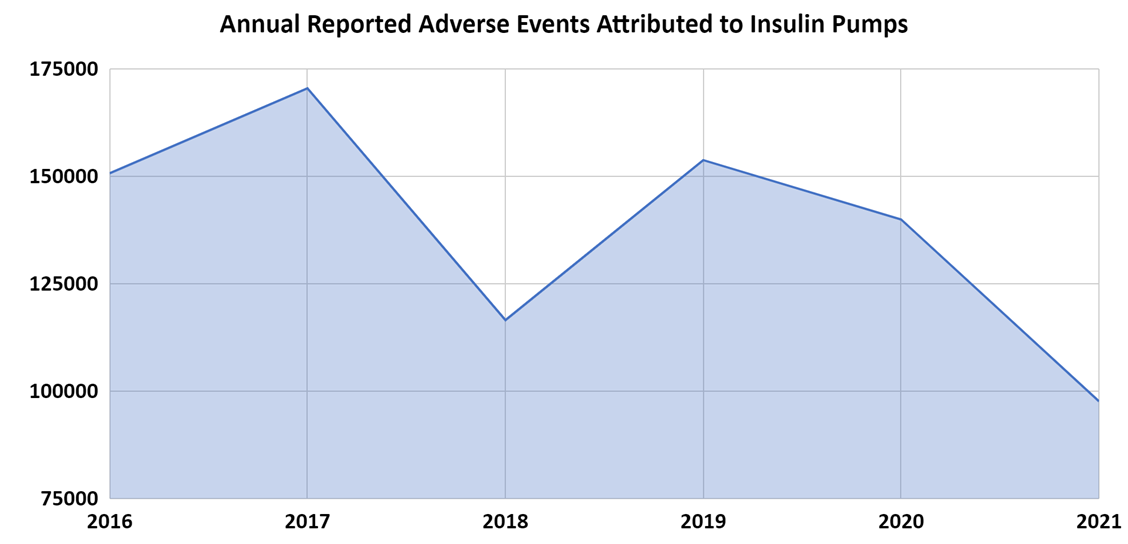
The MAUDE data indicate that total insulin pump adverse events increased 13.1% from 2016 to 2017 followed by a 31.6% decrease from 2017 to 2018. There was a temporary increase of 31.9% from 2018 to 2019, after which the decline continued 9% and 30.2% in the following years (Figure 4). Additionally, the MAUDE data indicate that total continuous glucose monitoring device adverse events steadily increased from 2016 to 2021 with a one-time decrease between 2018 and 2019. The annual adverse event totals increased from 111,428 in 2016, to 361,071 in 2021, which constitutes an overall increase of 224% (Figure 5).
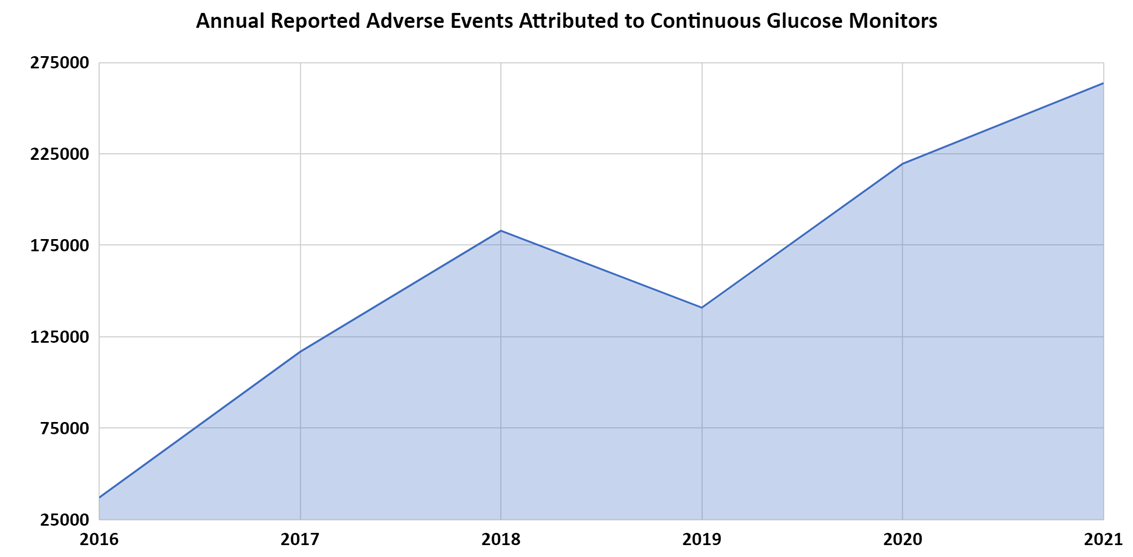
Considering the steadily increasing number of diabetes patients, averaging 1.5 million new cases per year, one would expect the use of primary treatment (i.e., insulin) and related devices (i.e., insulin pumps and continuous glucose monitors) to steadily increase as well. Further, despite increasing costs of insulin, one would expect insulin use to continue to increase since the medication is so critical to the survival of diabetic patients. However, comparing these trend lines, we can observe some counterintuitive phenomena that need further analysis.
Insulin adverse events appear to have peaked in 2018 and have generally been declining since. Insulin pump adverse events appear to have peaked in 2017 with a secondary peak in 2019 and have been generally declining as well. These two trends are not consistent with the steady growth in diabetes cases every year. Meanwhile adverse events related to continuous glucose monitors appear to be steadily going up every year, which would be the expected trend, along with the increase in the number of patients and the popularity of these devices which have surpassed $3 billion in sales annually.
Potential limitations of the study are that the FDA FAERS database relies on reports of adverse events from the pharmaceutical industry, healthcare providers, and patients. The number of reports may not reflect the actual adverse event rate. Duplicate and incomplete reports exist in the FAERS database, the existence of a report does not establish causation, and the information in the reports has not been verified. However, this study indicates overall trends in insulin adverse events, which may signal a decline in insulin utilization in the United States.
4. Conclusion
Since insulin costs have risen significantly over the years, it is possible that this may have caused certain low-income patients to reduce their own insulin use commensurate with their budgets. If this is the case, the long-term health consequences and the societal impact will be significant and it merits further study.
Another possibility is that, with increasing insulin costs, perhaps more patients are finding ways to directly buy insulin from countries such as Canada. For instance, as stated in a 2018 RAND corporation report, insulin costs in Canada are over 80% lower than those in the United States [10].
A more intriguing explanation of reduced insulin usage could be attributed to the steadily rising popularity of continuous glucose monitoring devices. One can surmise that frequent glucose readings may enable diabetes patients to notice smaller changes in their glucose levels, thus taking behavioural action before insulin may be needed. If this is the case, these devices are not merely offering convenience but are also impacting overall demand which may influence the economics of insulin. This possibility also merits further investigation.
Given the heavy burden of diabetes, both in the United States and worldwide, the observed trends require closer examination. Future research should quantify the number of insulin prescriptions filled in the United States, as well as hospitalization rates for patients resulting from diabetes complications.
References
[1] “Long-term Trends in Diabetes.” Centers for Disease Control and Prevention. (accessed April 3, 2022). View Article
[2] “National Diabetes Statistics Report 2020.” Centers for Disease Control and Prevention. (accessed April 3, 2022). View Article
[3] D. K. Roberts. “The Deadly Costs of Insulin.” American Journal of Managed Care. (accessed April 3, 2022). View Article
[4] I. B. Hirsch, “Introduction: History of Glucose Monitoring,” in Role of Continuous Glucose Monitoring in Diabetes Treatment. Arlington, VA, USA: American Diabetes Association, 2018, ch. 1, pp. 1. View Article
[5] “United States Blood Glucose Monitoring Devices Market Report 2021-2026 - Self Blood Glucose Monitoring Devices v/s Continuous Glucose Monitoring Devices.” PR Newswire. (accessed April 3, 2022). View Article
[6] B. H. McAdams and A. A. Rizvi, “An Overview of Insulin Pumps and Glucose Sensors for the Generalist,” Journal of Clinical Medicine, vol. 5, no. 1, Jan. 2016, doi: 10.3390/jcm5010005. View Article
[7] Sjö. “File: BGM twopart.JPG.” Wikimedia Commons. (accessed April 3, 2022) View Article
[8] “FDA Adverse Events Reporting System (FAERS) Public Dashboard.” U.S. Food & Drug Administration. (accessed April 3, 2022). View Article
[9] “MAUDE - Manufacturer and User Facility Device Experience.” U.S. Food & Drug Administration. (accessed April 3, 2022). View Article
[10] A. W. Mulcahy, D. Schwam and N. Edenfield. “Comparing Insulin Prices in the United States to Other Countries.” RAND Corporation. (accessed April 3, 2022). View Article
[11] “Diabetes mellitus.” Encyclopaedia Britannica. (accessed August 22, 2022). View Article
[12] “Medication & Treatments.” American Diabetes Association. (accessed August 22, 2022). View Article
[13] L. Heinemann, “Finger Pricking and Pain: A Never-Ending Story,” Journal of Diabetes Science and Technology, vol. 2, no. 5, Sep. 2008, doi: 10.1177/193229680800200526 View Article
[14] “Devices & Technology.” American Diabetes Association. (accessed August 22, 2022). View Article

