Volume 8 - Year 2021 - Pages 28-35
DOI: 10.11159/jbeb.2021.004
The Effect of Sterilization on the Bovine Pericardium Scaffold Decellularized By the Glutaraldehyde-Free Technology
Nataliia V. Shchotkina1,2,3, Anatoliy A. Sokol1, Liudmyla V. Dolinchuk2, Oleksandr Yu. Galkin2, Glib I. Yemets1, Dmytro A. Grekov1,2, Arkadii A. Dovghaliuk1, Iryna M. Skorokhod3, Olena V. Shepeleva2, Nadiia M. Rudenko1, Iliia M. Yemets1
1Center for Pediatric Cardiology and Cardio Surgery, Research laboratory, 24, Yurii Ilyenko street, Kyiv, Ukraine, 04050, info@cardio.org.ua
2 National Technical University of Ukraine “Igor Sikorsky Kyiv Polytechnic Institute", Department of Translational Medical Bioengineering, 37, Prosp. Peremohy, Kyiv, Ukraine, 03056, mail@kpi.ua
3XPand LLC, 40D, Kvitky Cisyk street, Kyiv, Ukraine 01014
nataliia.shchotkina@xpandmed.com.ua
Abstract - The use of xenotissue for the needs of regenerative and cardiovasculare medicine is a promising area of tissue engineering. The decellularization process provides complete purification of the elastin-collagen matrix of the bovine pericardium from cells and their components. The use of high concentrations of sodium dodecyl sulfate and glutaraldehyde can lead to a damage of the extracellular matrix. Therefore, the purpose of this study was to study the microarchitectonics of the decellularized matrix using a low concentration of sodium dodecyl sulfate (0.1% solution) and avoiding glutaraldehyde. Further stabilization and fixation of the matrix was carried out using 10 mM 1-Ethyl-3 (3-dimethylaminopropyl) carbodiimide hydrochloride and 10 mM N-Hydroxysuccinimide. The effect of decellularization was assessed by staining the samples with hematoxylin-eosin and by scanning electron microscopy. Also the research results confirmed the absence of structural changes in the collagen-elastin fibers of the matrix after sterilization dose of 10 kGy. Thus, it can be assumed that the radiation method of sterilization may be safe in use for sterilization of bioimplants.
Keywords: Tissue engineering, Cardiac bioimplant, Gamma sterilization
© Copyright 2021 Authors This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2021-09-30
Date Accepted: 2021-10-04
Date Published: 2021-11-22
1. Introduction
Tissue engineering has been recognized as a promising alternative to donor tissues, which often are in short supply. The concept of extracellular matrix (ECM) isolation of cells from native tissues and genetic material in order to produce a natural scaffold is a main goal of the decellularization. Clinically, ECM of biological tissues has been used in manufacturing heart valve prostheses, small-diameter vascular grafts, and biological patches [1-3]. The scientific literature confirms the effective use of ionic detergent SDS for decellularization process. The matrix is purified via solubilization of cytoplasmic and nuclear membranes, denaturation of proteins and removal of nuclear residues [4, 5]. However, these ECM have to be fixed with a crosslinking agent and subsequently sterilized before the implantation to humans [6]. Crosslinking can enhance the mechanical strength and reduce the immunogenicity of implanted grafts. In this study, we evaluated the effects of sterilization on the microstructure of decellularized bovine pericardium.
2. Materials and Methods
2.1. The procedure for tissue obtaining.
The material for the study was the bovine pericardium (BP). The pericardial sac was extracted from outbred 12–18 month old bulls after slaughter at the “Antonivskii Meatplant Ltd.”. The research was performed in accordance with the General Ethical Principles of Animal Experiments (Strasbourg, France, 1985) and Law of Ukraine No. 3447 - IV On Protection of Animals from Cruel Treatment (2006, edited in 2009). The biomaterial was transported to the laboratory during one hour in sterile Hanks solution in a container on ice. Pericardial sacs were dissected and non-fibrous components were removed. The tissue samples were cut in 40×40 mm. Samples were placed in distilled water in a volume of 1 000 ml and stirred continuously (70 rpm) for 3 hours at 4 °C.
2.2. Glutaraldehyde-free decellularization of the bovine pericardium.
BP samples were decellularized as follows: (1) osmotic shock was caused by placing samples into sterile distilled water (5 pieces per 500 ml of the solution) at 4ºC for 72 hours (200 rpm). Water was changed every 6 - 8 hours; (2) decellularization with 100 ml of 0.1% solution of SDS (Sigma-Aldrich, USA) with constant shaking (200 rpm) for 35 days at 4 °C; (3) washed with sterile NaCl solution for 7 days at 4 °C with constant shaking at 200 rpm; (4) stabilization and fixation in a solution of 70 % ethanol for 24 h at t 4 °C with constant shaking at 200 rpm; (5) washed with sterile NaCl solution for 24 h at 4 °C with constant shaking at 200 rpm; (6) cross-linking method - EDC/NHS solution – MES (10 mM 1-Ethyl-3- (3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), 10 mM N-Hydroxysuccinimide) and MES solution (pH 5.6) (0.05 M 2–morpholinoethane sulfonic acid); (7) washed with sterile NaCl solution for 24 h at 4 °C with constant shaking at 200 rpm. Non decellularized pericardial tissues were chosen as control samples.
2.3. Histological analysis.
Segments of non-decellularized (n=10) and decellularized (n=10) pericardial tissues were fixed in 10% neutral buffered formalin (Sigma-Aldrich, USA) for 1 h, embedded in paraffin, cut into 5-μm sections and stained with hematoxylin & eosin (H&E, Sigma Aldrich, USA). The stained samples were examined with Olympus BX 51 light microscope (Tokyo, Japan).
2.4. Scanning electron microscopy (SEM).
The samples of decellularized pericardium were dried by lyophilization at a vacuum depth of 30-50 Pa and a temperature of -50 ° C. (JFC-1100, Jeol, Tokyo, Japan). In order to eliminate the accumulation of surface charge during scanning with an electron beam in a microscope column, a thin layer of gold was applied to the dried samples by cathodic sputtering (JFC-1100, Jeol, Tokyo, Japan). The preparations were investigated in SEM JSM 6060 LA (Jeol, Tokyo, Japan) in the secondary electron mode at an electron acceleration voltage of 30 kV.
2.5. Sterilization
Sterilization was performed physically. Irradiation of 10 kGy at a temperature of +20 for 8 minutes.
2.6. Sterility control
The test is intended to determine whether the sterilization method used satisfies the appropriate level of microbiological purity of the test material.
For this purpose, a standard operating procedure (hereinafter - SOP) was used, which determines the sequence of actions during sterility control and is intended for use in the microbiological research sector.
The tests were performed using the method of direct seeding. Sowing is carried out in the biosafety box AC4-4E8 Airstream, Class 2. Appropriate negative control experiments were performed.
Under aseptic conditions, the package was opened and the test matrix samples were cut into pieces, which were immersed in appropriate nutrient media.
The cultures were incubated for 14 days at a temperature of 30 ° C ± 2.5 ° C - Thioglycol broth and at a temperature of 22 ° C ± 2.5 ° C - Saburo broth. The temperature of the incubators was monitored and recorded daily. In parallel with the experimental samples are placed unsown tubes to control the sterility of the medium. Crops were inspected daily, noting the transparency of the environment - the lack of growth. If there is growth - made fixed smears and looked under a microscope.
2.7. Statistical analysis.
The analysis of the research results was carried out using biostatistical methods [7]. For quantitative values, the normality distribution was analyzed using the Shapiro-Wilk test. The mean value (M) and standard deviation (± SD) were calculated. To estimate the mean value, its 95% confidence interval (95% CI) was calculated. For qualitative values, the frequency (%) and, if necessary, 95% CI were calculated. When comparing quantitative parameters in more than two groups, one-way ANOVA was used [7], posteriori comparisons were carried out using Scheffe Test (The distribution law did not differ from normal). To compare qualitative parameters, the chi-square test was used, posteriori comparisons for more than two groups were carried out taking into account the Bonferroni correction [7-9]. In the analysis, the criteria for a bilateral critical area were used, the critical level of significance was 0.05. Statistical analysis of the research results was carried out in the statistical package EZR v. 1.54 (Saitama Medical Center, Jichi Medical University, Saitama, Japan, 2020) [10], which provide a graphical interface to R (The R Foundation for Statistical Computing, Vienna, Austria).
3. Results
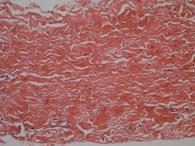
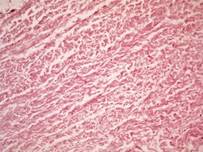
The use of sodium dodecyl sulfate (SDS) in the decellularization process can significantly affect the structure of the matrix collagen fibers [11, 12]. Therefore, one of the main tasks was to conduct a study on the morphological assessment of the structure of matrix fibers that had already undergone the processes of decellularization and stabilization by the cross-linking method without glutaraldehyde. The use of a histological method makes it possible to assess the degree of matrix purification from cells and the degree of structural changes in collagen fibers. As a control, we used the native pericardium of bovine, in the form of a narrow plate with thick collagen and thin elastin fibers, and with densely formed connective tissue. In a sample of the native pericardium, a small amount of fusiform fibroblasts with rod-shaped, moderately basophilic nuclei and weakly basophilic cytoplasm were registered (Figure 1, a). The structure of the collagen resembled tightly twisted bundles, which were located in a parallel direction to each other. This fiber architectonics provides pericardial resistance to mechanical stress.
In turn, it should be noted that this effect depends on the dose and time of exposure.In the Experimental technology for obtaining a decellularized extracellular matrix the effect of complete purification from cells and components was observed after 21 days of decellularization (Table 1). In the samples of this technology, the structure of the pericardium was similar to the native tissue. Collagen fibers were denser with no space between bundles. A decrease in the sinuosity of fibers inherent in native tissues was noted, and in some areas, on the contrary, the amplitude of fiber bending increased. The bundles resembled thick strands, between which spaces with thin fragmented fibers were locally formed (Figure 1, B).
Table 1. Histological analysis of cell detection in decellularized by various technologies matrix, hematoxylin-eosin staining method
|
Technologies |
Cells, (%) |
p |
|||
|
No cells in the sample |
Single cells in the sample |
A small number of cells |
A large number of cells |
||
|
Control (native bovine pericardium) (n=25) |
0 (0,0) |
0 (0,0) |
0 (0,0) |
25 (100,0) |
<0,001 |
|
Experimental Technology (n=25) |
24 (96,0) |
1 (4,0) |
0 (0,0) |
0 (0,0) |
|
Notes: the chi-square criterion was used for comparison between groups, posterior comparisons were made taking into account the Bonferroni correction.
The analysis revealed a statistically significant difference between the groups in terms of the parameter expression degree (p <0.001 according to the chi-square test). At the same time, the parameter expression degree for the Control group samples (cattle native pericardium) was statistically significantly (p <0.05) higher than for the samples of the Experimental Technology. It should be emphasized that for the Control group (native bovine pericardium) “no cells” was noted in 0% (95% CI 0.0% - 7.4%) of samples, and for Experimental Technology - in 96.0% (95% CI 84.3% - 100%) samples.
 B
B 
Qualitative microstructural analysis of SEM images also confirmed the absence of obvious changes in the structure and distribution between the collagen and elastin fibers of the decellularized extracellular matrix of the bovine pericardium (Figure 2). The fibers are compactly arranged in relation to each other. Also SEM showed that collagen fibers were reticulated in decellularized bovine pericardium after crosslinked by EDC/NHS and relative aperture of the collagen fiber was from 10 to 20 μm. Collagen fibers did not significantly change their orientation after the effect of Gamma sterilization of dose 10 kGy and their curves were similar to samples of decellularized bovine pericardium (Figure 3).
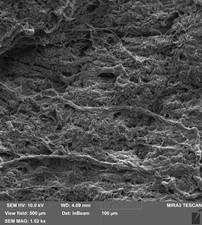
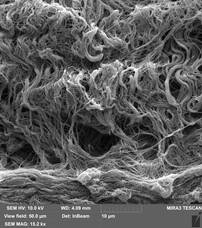

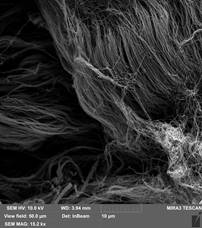
 B
B 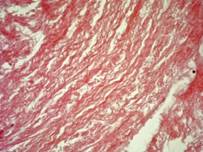
Data showed that in the decellularized bovine pericardium at a dose of 10 kGy of electron beads the number (%) of collagen fibers per test area of the samples after sterilization was almost no different from the samples that did not undergo the sterilization step. Therefore, the percentage of collagen fibers was 95,5± 0,7 and 98,2± 0,3, respectively.
However, it should be noted that the changes in structure and orientation were more pronounced, although they did not reach a significant difference.Violations of the integrity and uniformity of collagen fiber curls were observed in 19±1,1 % of specimens that underwent sterilization and 8±0,7 % in specimens before sterilization, 2±0,5 % in comparison with native bovine pericardium, which did not pass the stages of decellularization and sterilization, respectively
Sterility control was determined after 14 days incubation of decellularized bovine pericardium in Thioglycol medium and Saburo medium. Thus, in the studied samples of decellularized bovine pericardium before sterilization, turbidity of both media was observed Fig. 5. (A), indicating the presence of both bacterial and fungal microflora. In turn, the media with the samples after sterilization remained transparent Fig. 5 (B and C), and therefore the growth of microflora was absent. Thus, it can be concluded that this mode of sterilization provides the desired result - the sterility of the test samples.
 B
B  C
C 
A - Presents of growth of microorganisms on samples of decellularized bovine pericardium before sterilization, Saburo and Thioglycollate medium, respectively. B - Absence of growth of microorganisms on samples of decellularized bovine pericardium before sterilization, Thioglycollate medium. C - Absence of growth of microorganisms on samples of decellularized bovine pericardium before sterilization, Saburo medium.
4. Discussion
The present study evaluates the effect of gamma sterilization on sterilization of ECM for xenotransplantation. We show that gamma sterilization is not deleterious for the decellularized pericardial tissue and allows safe collagen-elastin ultrastructure of scaffold. Decellularization is aimed at cleansing the collagen-elastin carcass from cells and their components. Such matrix can later be used as a cardiological patch to close defects of the cardiovascular tissue. It is important that the structure of the matrix would be as integral and strong as possible, due to the colonization with cells during biointegration into the donor tissue.
Different decellularization protocols have been used in the past with mixed success [1, 5]. Most of the protocols are based on the anionic detergent SDS, which is an effective decellularization agent and successfully applied in the decellularization of many tissues and whole organs [1-3]. However, many studies based on ECM ultrastructure report deleterious effects of SDS including reduction in collagen [11, 12]. Alterations in the structural composition of ECM during decellularization can affect cell attachment, differentiation and function. Reduced cellular functionality has been attributed to matrix alterations caused by SDS decellularization [13]. However, low concentrations of SDS were shown to have less matrix disruption on kidney decellularization when little or no damage in renal tubules and vessels and minimal disruption of glomeruli were observed [14]. In our study, we used 0.1% SDS solution, which removed almost all the cellular content of decellularized pericardium compared to the native pericardium control, without significantly disrupting the extracellular matrix. Our H&E staining confirmed the removal of the cellular component from the decellularized scaffold.
Usually after the process of decellularization the fiber structure is porous and stratified [6]. Therefore, an important step in tissue biotransformation is the final stage of stabilization. In the vast majority of commercial patches (scaffolds) glutaraldehyde is used for fixation. However, residual glutaraldehyde can lead to cytotoxicity, inflammation and calcification of the bioimplant with long-term observation [15]. In this study for the stabilizing the matrix fibers we used the soluble carbodiimide EDC (1-Ethyl-3- (3-dimethylaminopropyl) carbodiimide) and an organic substance derived from pyrrolidine NHS (N-hydroxysuccinimide), which are successfully used in medicine in the treatment of catarrh in vivo [16, 17]. EDC is the most popular zero-length crosslinker for biochemical conjugations because it can efficiently form conjugates between two protein molecules, between a protein and a peptide, and between proteins and oligonucleotides, and with small molecules.
Direct EDC mediated crosslinking can be done without EDC becoming part of the final amide bond between the target molecules. Another advantageous quality of EDC is that it is water soluble and dissolves in aqueous buffer solutions, like most biological macromolecules. Therefore, EDC and its by-product, isourea, are dissolved into the reaction medium allowing easy purification of the crosslinked product with precipitation, chromatography, dialysis, or ultrafiltration. For higher coupling efficiency and more stable amine-reactive intermediates, EDC crosslinking protocols often include N-hydroxysuccinimide (NHS) or its water-soluble analog (Sulfo-NHS). EDC, in conjunction with NHS allows, for 2-step coupling of two proteins without affecting the carboxyls of the second protein. First, EDC activates carboxyl groups and forms an amine reactive O-acylisourea intermediate that spontaneously reacts with primary amines to form an amide bond and an isourea by-product. The O-acylisourea intermediate is unstable in aqueous solutions and failure to react with an amine will cause hydrolysis of the intermediate, regeneration of the carboxyls, and the release of an N-substituted urea. Therefore, it is necessary to quench the EDC activation reaction with a thiol-containing compound like 2-mercaptoethanol. EDC couples NHS to carboxyls, which forms an NHS ester that is considerably more stable than the O-acylisourea intermediate and allows for efficient conjugation to primary amine.s at physiologic pH.
Microstructure analysis of SEM images in this study also confirmed that the micro-arhitectonic of the decellularized tissues by SDS and EDC/NHS cross-linking were not changed.
For further use of ECM in clinical practice - an integral stage of tissue processing is the process of sterilization of the material as a device. However, this stage of tissue treatment can also affect its biological properties, and methods that can be safely used to sterilize tissues are limited. Techniques that are the most commonly used for biomaterials sterilization include chemical treatment (ethanol, ethylene oxide), antibiotic treatment, irradiation techniques (ultraviolet irradiation, gamma and electron beam irradiation), and heat treatment. Each of the sterilization methods has its advantages and limitations.
The selection of an effective sterilization method is extremely important in order to avoid contamination of the samples, as well as undesirable changes in physical and chemical properties of the sterilized material [18, 19]. The aim of this study was to study influence of Gamma radiation sterilization technique in dose 10 kGy to collagen fibrous of scaffolds in the terms of their effectiveness, impact on scaffolds’ morphology and architectonic. Bosworth et al. in their work reported that, despite the lack of visible changes in the fibers morphology immediately after sterilization, degradation of the polylactic-co-glycolic acid (PLGA) membrane occurs much faster than the degradation of the non-sterile membrane [20]. A similar phenomenon was observed at work [21].
Gamma irradiation exposure might result in breaking polymer molecular chains into smaller
fragments, which leads to change in material properties and its degradation [22].
Although many authors reported gamma sterilization as an effective method for fibrous materials [20, 21], degradation of the material following sterilization could make this method unsuitable for materials for biomedical applications. In turn, it should be noted that this effect depends on the dose and time of exposure of radiation.
5. Conclusion
Histological and biomechanical data of the test showed that 0,1% SDS protocol is optimal for the procedure of decellularization of bovine pericardium. Studies have shown that pericardial tissue decellularized by low-concentration of 0.1% SDS saves the fiber architectonics and provides pericardial resistance to mechanical stress. The analysis revealed a statistically significant difference between the groups in terms of the parameter expression degree.
Thus, the study showed the effectiveness of using low concentrations of SDS (0.1%) and EDC/NHS cross-linking in the decellularization.
Based on the results of this studies, it can be concluded that the sterilization dose of 10 kGy did not have a significant effect on the structure and architecture of collagen fibers in decellularized bovine pericardium, while ensuring complete absence of bacterial and fungal microflora growth in the studied samples. Thus, it can be assumed that the radiation method of sterilization may be safe in use for sterilization of bioimplants.
References
[1] F. Naso, A. Gandaglia, “Different approaches to heart valve decellularization: A comprehensive overview of the past 30 years”. Xenotransplantation. vol. 25, no 1, pp. 1-10, 2018. View Article
[2] S.E. Gilpin, J.P. Guyette, G. Gonzalez, X. Ren, J.M. Asara, D.J. Mathisen, J.P. Vacanti, H.C. Ott, “Perfusion decellularization of human and porcine lungs: Bringing the matrix to clinical scale”, The Journal of Heart and Lung Transplantation., vol. 33, no 3, pp. 298-308, 2014. View Article
[3] R. Ramm, T. Goecke, K. Theodoridis, K. Hoeffler, S. Sarikouch, K. Findeisen, A. Ciubotaru, S. Cebotari, I. Tudorache, A. Haverich, A. Hilfiker, “Decellularization combined with enzymatic removal of N-linked glycans and residual DNA reduces inflammatory response and improves the performance of porcine xenogeneic pulmonary heart valves in an ovine in vivo model”. Xenotransplantation. 2020 Mar;27(2):e12571. DOI: 10.1111/xen.12571 View Article
[4] E. Rieder, M.T. Kasimir, G. Silberhumer, G. Seebacher, E. Wolner, P. Simon, G. Weigel, “Decellularization protocols of porcine heart valves differ significantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells”, J Thorac Cardiovasc Surg., vol. 127, no 2, pp. 399–405, 2004. View Article
[5] R. Simsa, A.M. Padma, P. Heher, M. Hellström, A. Teuschl, L. Jenndahl, N. Bergh, P. Fogelstrand, “Systematic in vitro comparison of decellularization protocols for blood vessels”. PLoS One. vol. 13, no 12, pp e0209269, 2018 DOI: 10.1371/journal.pone.0209269 View Article
[6] Xiu-Fang Xu1, Hai-Ping Guo, Xue-Jun Ren, Da Gong, Jin-Hui Ma, Ai-Ping Wang, Hai-Feng Shi, Yi Xin, Ying Wu, Wen-Bin Li1, “Effect of carbodiimide cross-linking of decellularized porcine pulmonary artery valvular leaflets”, Int J Clin Exp Med., vol. 7, no 3, pp. 649-656, 2014.
[7] V.G. Guryanov, Yu. E. Lyakh, V.D. Pariy, O.V. Korotky, O.V. Chaly, K.O. Chaly, J.V. Tcekhmister, Handbook of Biostatistics. Analysis of the results of medical research in the package EZR (R – statistics). Kyiv, Vistka, 2018.
[8] I.V. Nikolaenko, A.Iu. Galkin, G.E. Raevskaia, T.V. Kas'ianenko, M.I. Nereshchenko, E.S. Donskaia, N.Ia. Spivak, “Preparation of monoclonal antibodies to the Fc-fragment of human IgG and the use of their based immunoenzyme conjugates”, Klin Lab Diagn., vol. 11, pp. 8-11, 2005.
[9] I.V. Nikolaenko, V.S. Goncharenko, N.N. Shimko, A.Yu. Galkin, “Isolation of surface antigen of hepatites B virus”, Ukrain'skyi Biokhimichnyi Zhurnal, vol. 79, no 2, pp. 114-122, 2007.
[10] Y. Kanda, “Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics.”, Bone Marrow Transplant., vol. 48, pp. 452–458, 2013. View Article
[11] D.M. Faulk, C.A. Carruthers, H.J. Warner, C.R. Kramer, J.E. Reing, L. Zhang, A. D’Amore, S.F. Badylak, “The effect of detergents on the basement membrane complex of a biologic scaffold material”, Acta Biomater., vol. 10, no 1, pp. 183–193, 2014. View Article
[12] H. Ren, X. Shi, L. Tao, J. Xiao, B. Han, Y. Zhang, X. Yuan, Y. Ding, “Evaluation of two decellularization methods in the development of a whole-organ decellularized rat liver scaffold”, Liver international: official journal of the International Association for the Study of the Liver. vol. 33, no 3, pp. 448–458, 2013. View Article
[13] O. Syed, N.J. Walters, R.M. Day, H.W. Kim, J.C. Knowles, “Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering”, Acta Biomater., vol. 10, no 11, pp. 5043–5054, 2014. View Article
[14] M. Caralt, J.S. Uzarski, S. Iacob, K.P. Obergfell, N. Berg, B.M. Bijonowski, K.M. Kiefer, H.H. Ward, A. Wandinger-Ness, W.M. Miller, Z.J. Zhang, M.M. Abecassis, J.A. Wertheim, “Optimization and Critical Evaluation of Decellularization Strategies to Develop Renal Extracellular Matrix Scaffolds as Biological Templates for Organ Engineering and Transplantation”, Am J Transplant., vol. 15, no. 1, pp. 64–75, 2015. View Article
[15] H. Shang, S.M. Claessens, B. Tian, G.A. Wright, “Aldehyde reduction in a novel pericardial tissue reduces calcification using rabbit intramuscular model. Journal of materials science”, Materials in medicine., vol. 28, no 1, pp. 1-16, 2017. View Article
[16] G. Wollensak, E. Spoerl, T. Seiler, “Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus”, Am J Ophthalmol., vol. 135, no 5, pp. 620-627, 2013. View Article
[17] O.V. Shtapenko, I.I. Hevkan, Yu.I. Slyvchuk, V.Y. Syrvatka, N.M. Matvienko, “Formation and properties polymer nanolayers to enhance cell growth in vitro”, Innovative Biosystems Bioengineering, vol. 2, no 2, pp. 105-109, 2018. View Article
[18] Rediguieri, C.F.; Sassonia, R.C.; Dua, K.; Kikuchi, I.S.; de Jesus Andreoli Pinto, T. Impact of sterilization methods on electrospun scaffolds for tissue engineering. Eur. Polym. J. 82, 181–195, 2016. View Article
[19] Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 7, 2016. View Article
[20] Bosworth, L.A.; Gibb, A.; Downes, S. Gamma Irradiation of Electrospun Poly (e-caprolactone) Fibers Affects Material Properties but Not Cell Response. J. Polym. Sci. Part B Polym. Phys. 50, 870–876, 2012. View Article
[21] Selim, M.; Bullock, A.J.; Blackwood, K.A.; Chapple, C.R.; MacNeil, S. Developing biodegradable scaffolds for tissue engineering of the urethra. BJU Int. 107, 296–302, 2011. View Article
[22] Gorna, K.; Gogolewski, S. The effect of gamma radiation on molecular stability and mechanical properties of biodegradable polyurethanes for medical applications. Polym. Degrad. Stab. 79, 465–474, 2003. View Article

