Volume 12 - Year 2025 - Pages 07-17
DOI: 10.11159/jbeb.2025.002
BCI in Stroke Rehabilitation with Robotic Devices, tACS and FES: A Case Study
Teodiano Bastos-Filho1, Aura Ximena González-Cely1, Sheida Mehrpour1, Sheila Schreider1, Fernanda Souza1, Fernando Cabral1, Ana Cecilia Villa-Parra2
1Universidade Federal do Espírito Santo
Av. Fernando Ferrari, 514, Vitoria, ES, Brazil 29075-910
teodiano.bastos@ufes.br; sheyda.mehrpour@gmail.com; sheiladaluz@gmail.com; fernandavaz.souza@hotmail.com;
fernando.cabral@edu.ufes.br
2Universidad Politécnica Salesiana, Cuenca, Ecuador 010150
avilla@ups.edu.ec
Abstract - This work presents the application of a rehabilitation protocol using a Brain-Computer Interface (BCI) based on Motor Imagery (MI) and Neurofeedbak (NF), and applying transcranial Alternating Current Stimulation (tACS) and Functional Electrical Stimulation (FES) together with the use of robotic devices, such as a robotic monocycle and a robotic glove. This protocol uses the concept of Alternating Treatment Design (ATD), in which a single chronic post-stroke patient is submitted to these techniques. The rehabilitation progress was analysed through EEG and clinical metrics, such as Fugl-Meyer Assessment Scale (FMS), Functional Independence Measurement (FIM), Modified Ashworth Scale (MAS), MiniBESTest (MBT), modified Rankin Scale (mRS), Time Up and Go (TUG), 10-Meter Walk Test (10MWT), National Institutes of Health Stroke Scale (NIHSS), Barthel Index (BI), and surface electromyography (sEMG). Results from these metrics include 6% increase in Fugl-Meyer Assessment Scale (FMS) for upper-limb and 9% increase for lower-limb; 8% increase in Functional Independence Measurement (FIM), and an improvement in the FIM score from 5.83 to 6.27; 25% increase in MiniBESTest (MBT); 30% decrease in Time Up and Go (TUG); 18% increase in time and 25% increase in number of steps for the 10-Meter Walk Test (10MWT); 25% decrease in NIHSS; and 14% increase in BI. For the metrics MAS and mRS, there was not variation, with MAS maintaining the Grade 1, and mRS maintaining a score of 3. Regarding the results for surface Electromyography (sEMG), there was a 5% increase in muscle contraction peak for finger flexors, 2% for tibialis anterior and 21% for rectus femoris. For the EEG analysis, topographic maps showed increase of energy ratio in beta/mu beta rhythms at the end of intervention.
Keywords: Brain-Computer Interface, Functional Electrical Stimulation, Neurorehabilitation, Robotic Devices, Stroke, Transcranial Alternating Current Stimulation.
© Copyright 2025 Authors This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2025-04-11
Date Revised: 2025-08-28
Date Accepted: 2025-09-15
Date Published: 2025-10-21
1. Introduction
A study by the United Nations forecasts that, by 2050, the population of individuals aged 65 and older will reach 1.5 billion, constituting 16% of the total population [1]. This demographic increase will be accompanied by an increase in age-related health concerns, notably stroke, as the likelihood of experiencing a stroke double roughly every 10 years after turning 55 [2]. Stroke is a clinical condition marked by insufficient blood flow to the brain, which leads to cell death, whose impact largely depends on the location and its severity. Stroke can be ischemic (80% of cases), caused by reduced blood flow, or haemorrhagic, due to bleeding. Individuals recovering from a stroke may face varying degrees of neural injury affecting motor functions in both upper- and lower-limbs [3].
The effects of a stroke on the upper-limb can be a weakness (hemiparesis) or paralysis (hemiplegia) on one side of the body (often seen on the opposite side to where the stroke occurred), as well as loss of fine motor skills of fingers and hands; spasticity (increased muscle tone or stiffness in the affected arm), sensory changes (such as numbness, tingling, or a "pins and needles" sensation), and difficulty with the coordination of shoulder, elbow, wrist, and fingers [3]. On the other hand, the lower-limb effects can be weakness or paralysis (similar to the upper-limb) in one leg, affecting the ability to walk and perform weight-bearing activities; impaired balance and coordination while standing and walking, increasing the risk of falls; spasticity (due to tightness and stiffness in the muscles of the affected leg, making walking and movement challenging); foot drop (the foot cannot be lifted properly due to weakness or paralysis of the muscles that control dorsiflexion (lifting the foot upwards), which can lead to dragging the foot while walking; and altered gait patterns (such as a limp, scissoring gait – legs crossing over each other –, or circumduction – swinging the leg out to the side while walking) [3]. After a stroke, rehabilitation for impairments in both the upper- and lower-limbs typically consists of a blend of physical therapy, occupational therapy, and sometimes speech therapy, whose goal is to improve strength, range of motion, coordination, and functional abilities to enhance independence in Activities of Daily Living (ADL) [4], [5].
Early intervention is vital for optimal outcomes in upper- and lower-limb rehabilitation, capitalizing on the brain's adaptability known as "neuroplasticity" (neuro system’s ability to adapt and reorganise its structure in reaction to stimuli) [6], [7]. Traditional therapies focus on strength, range of motion, motor control, and ADL performance [8]. Such therapies form the foundation, but advancements like technology-enhanced therapies offer new possibilities for optimizing training intensity, repetition, progress evaluation, and patient-specific treatment [6], [8] .
Recent years have shown innovations that can transform post-stroke rehabilitation, such as the use of Robot-Assisted Training (orthoses, exoskeletons, pedaling devices), Brain Stimulation, Neuromodulation, Muscular Electrical Stimulation, Virtual Reality and Serious Games, Surface EMG (sEMG), Machine Learning / Artificial Intelligence, Neurofeedback which provides feedback and tracks progress for personalized rehabilitation [9], [10], [11] with positive outcomes in the field of rehabilitation. Fig 1 summarizes the technologies reported in the literature as emerging paradigms to support stroke survivors.
Novelties in this field are the use of Functional Electrical Stimulation (FES) that induces peripheral activation to enhance muscle contraction, through suitable pulse amplitude, duration and frequency, to generate stimuli triggering action potentials in intact peripheral nerves [9],
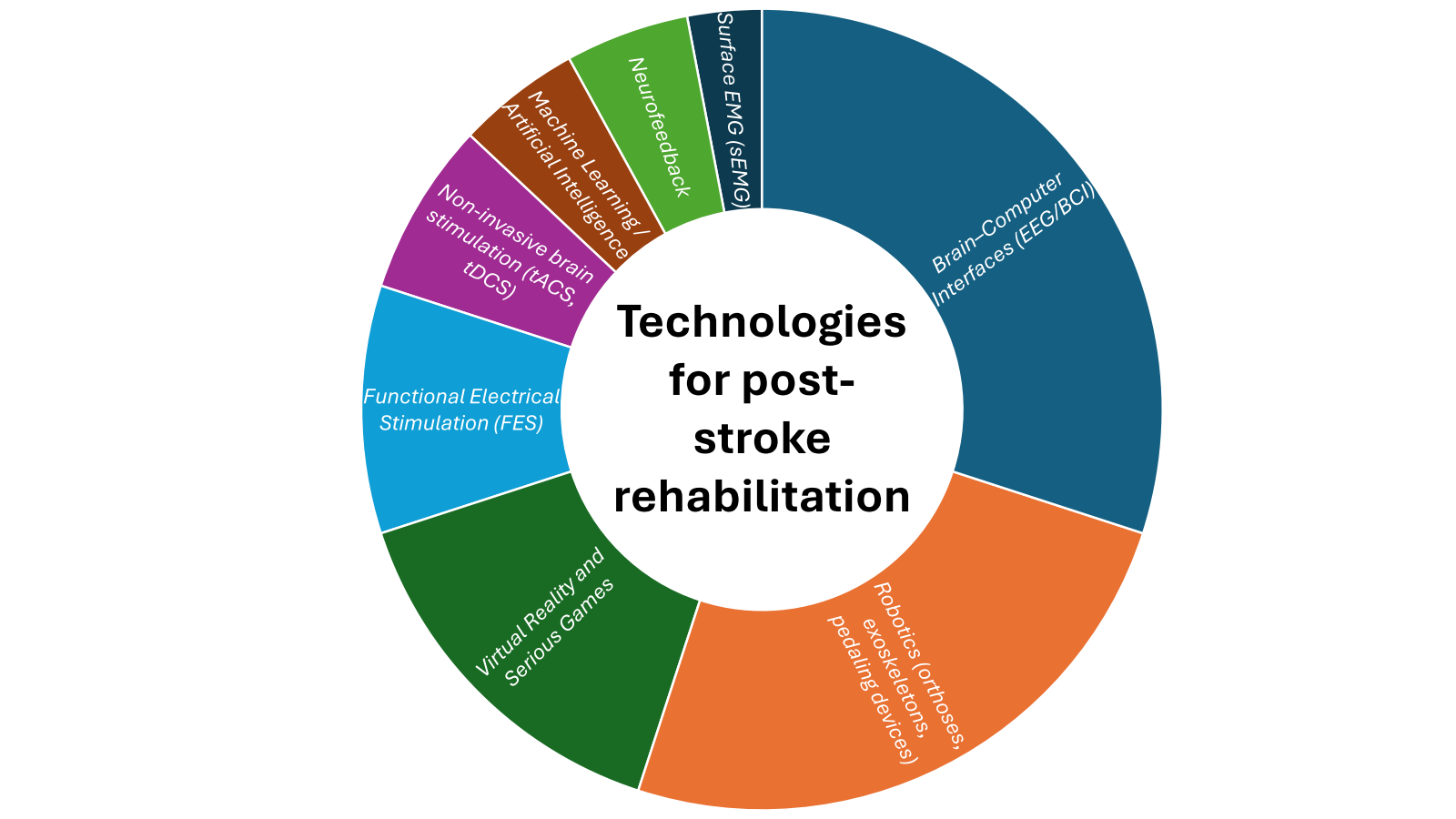
Non-invasive brain stimulation (tACS, tDCS) that regulates brain oscillations and reshapes brain rhythms [10]. Rehabilitation involving a Brain-Computer Interface (BCI) is another innovation that provides a groundbreaking approach. In fact, BCI establishes direct communication between the brain and the rehabilitation equipment [6], [11]. Machine learning models predict functional recovery outcomes and dynamically adjust therapy intensities. [11] Moreover, studies have shown that BCIs based on Motor Imagery (MI) of upper- and lower-limb movements can improve motor learning and increase neural plasticity [12], [13]. These motor patterns can be analysed through Event-Related Synchronization (ERS)/Desynchronization (ERD), which are associated to indicate neural changes, specifically a power increase or decrease in the brain’s electrical signals, respectively [14], [15]. Thus, BCIs can translate these detected MI into commands for external devices, enhancing motor function and independence for stroke survivors [5]. As part of such MI-BCI therapy, the inclusion of Neurofeedback (NF) has shown improvements in neuroplasticity, as this technique measures and shows to the patient their brain wave activity to help them self-regulate their brain function and enhance specific targets [16].
2. Materials and Methods
Figure 2 shows the methodology used in our study for upper- and lower-limb post-stroke rehabilitation through a BCI, robotic devices, tACS and FES.
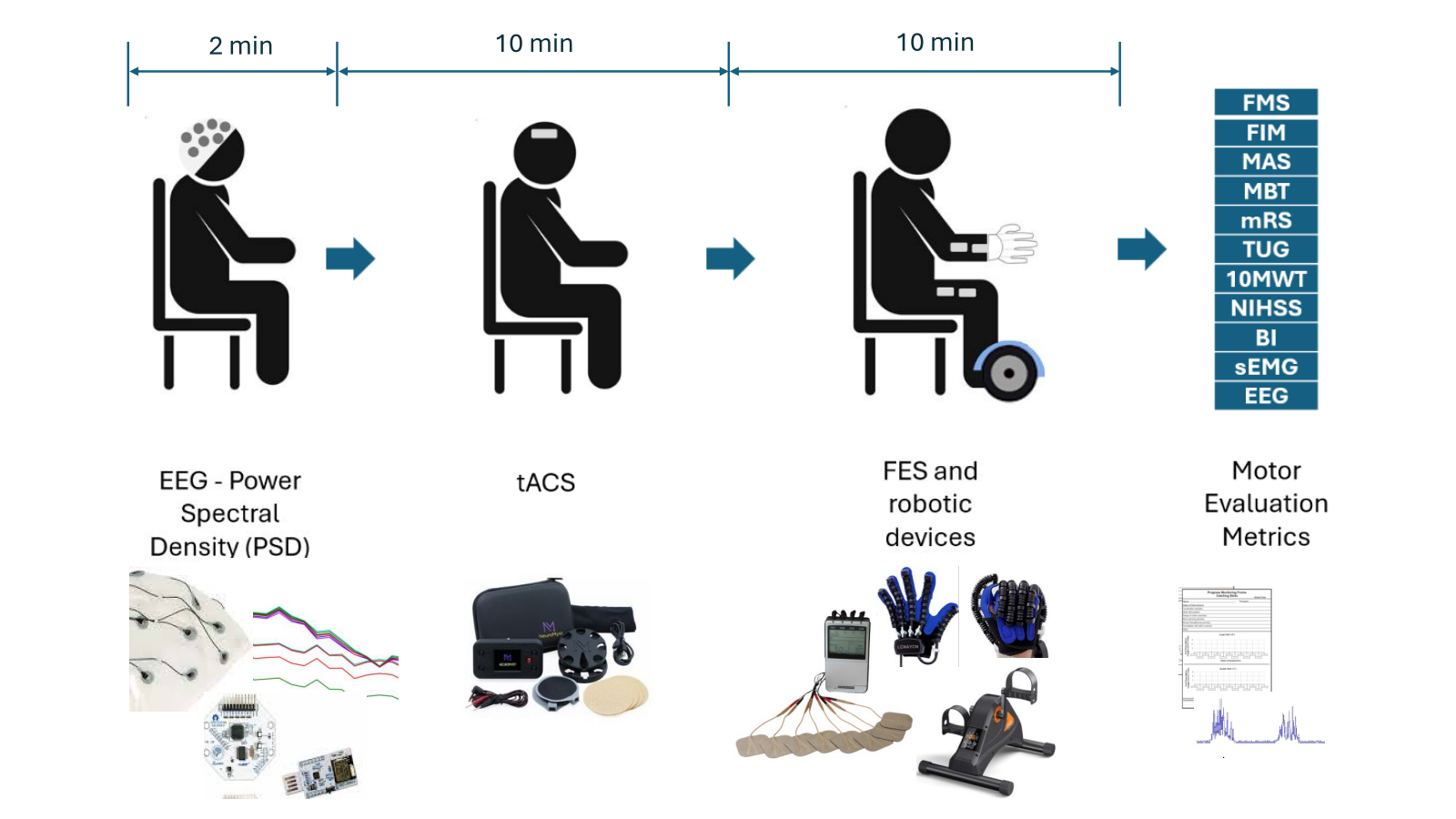
This protocol was developed through a literature review and expert consultation in neurology, rehabilitation, and engineering. It integrates biosignal-controlled robotic technology to capture patient intent and stimulation technologies to enhance motor activity. Evaluation is based on standard rehabilitation metrics.
2.1 Patient characteristics
The patient of our study is a 64-year-old man who had an ischemic stroke in October 2024, small vessel type, according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification [17]. His risk factor was non-treated hypertension. Computed Tomography (CT) scan of his brain showed a lacunar ischemic stroke in the left capsular nucleus region. His electrocardiogram exam showed sinus rhythm, the presence of atrioventricular conduction disturbance, and non-sustained ventricular tachycardia. His echocardiogram showed no abnormalities. The patient was recruited for this study in February 2025.
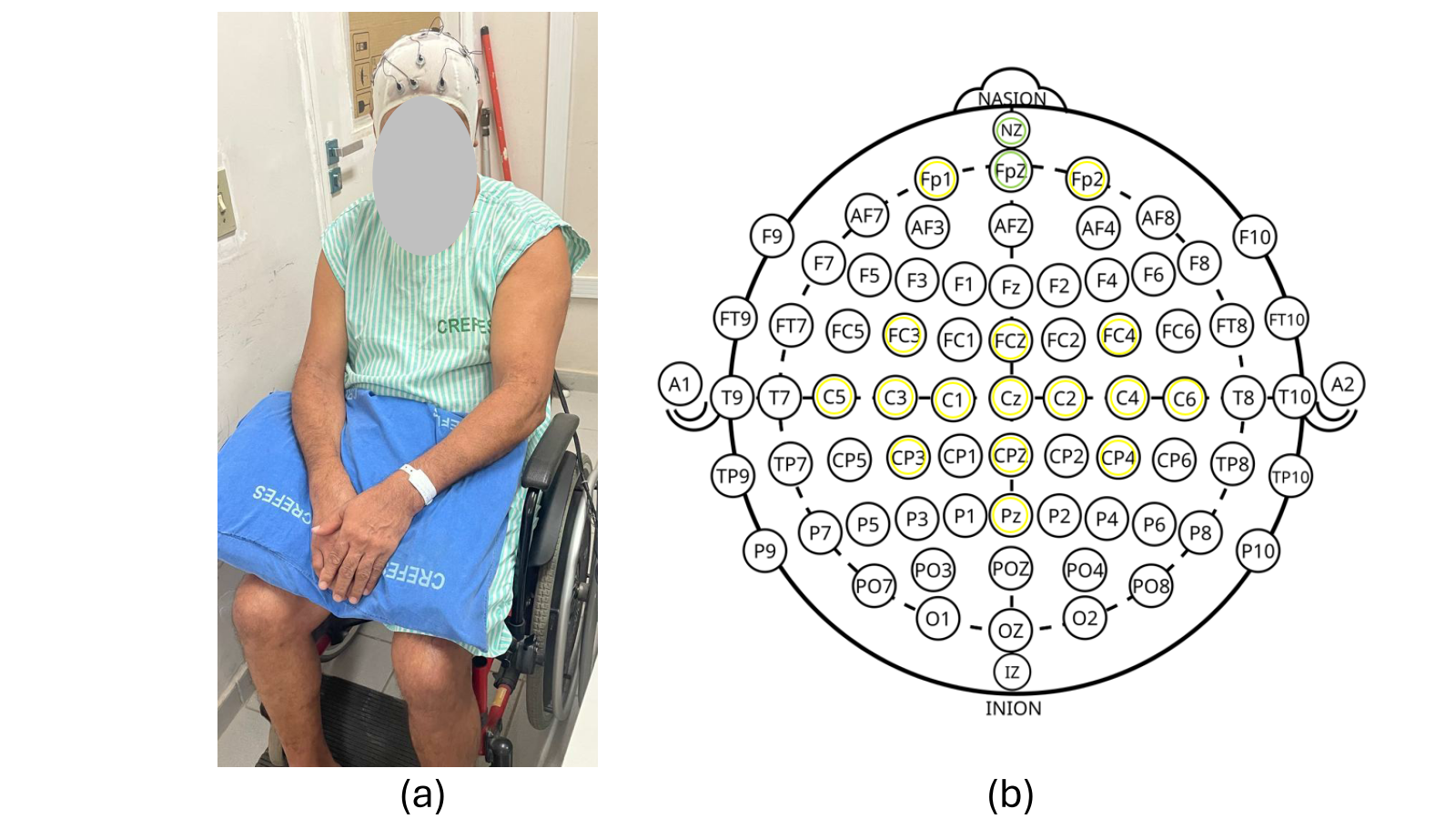
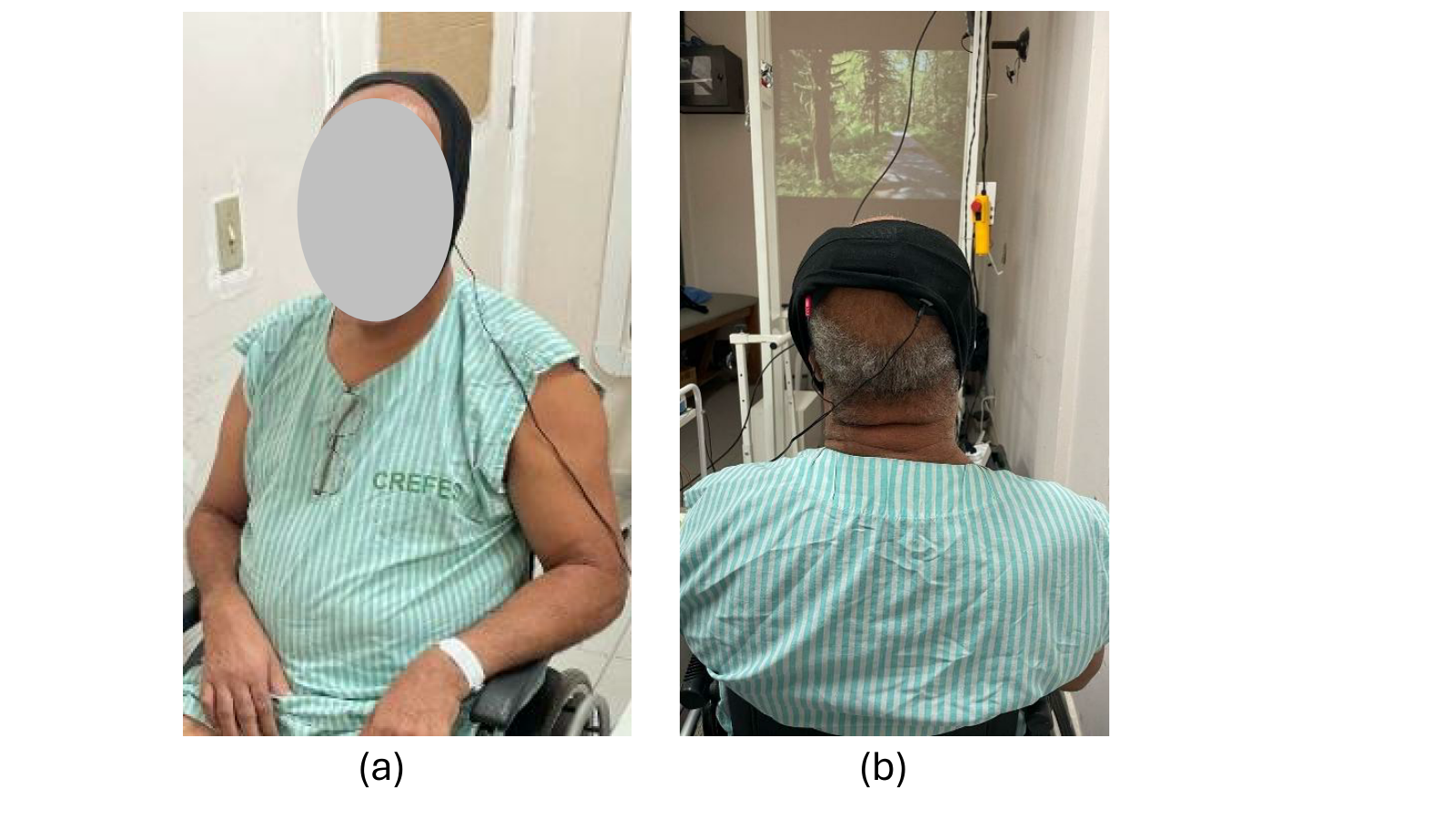
Figure 3(a) shows the patient wearing the EEG cap, which is used to recognize MI patterns when imaging limb movements, and Fig 3(b) shows the electrodes used to acquire the EEG signals.
2.2 Measuring MI frequencies
To acquire EEG signals, wireless signal acquisition boards (Cyton-Daisy, OpenBCI, USA) were used, which sample at 125 Hz signals from sixteen EEG electrodes located on: Fp1, Fp2, FC3, FCz, FC4, C5, C3, C1, Cz, C2, C4, C6, CP3, CPz, CP4, and Pz, and with two references at Nz and Fpz, such as shown in Fig 3(b).
Cortical activity during MI and MI+NF conditions was analysed through Relative Power Changes (P) in the mu (8–12 Hz) and beta (18–24 Hz) bands. The value of P for each electrode was computed in 1-s windows based on Fourier Fast Transform (FFT) values for these bands. EEG signals were processed in real time, using a 1-s window with 500 ms overlap for data segmentation, such as done in [18], [19], [20], [21]. Preprocessing included Laplacian Average Reference (LAR) filtering to reduce interference at electrodes C3 and C4, along with a 4th-order zero-phase Butterworth band-pass filter (7–30 Hz). The normalized power band, , was calculated for each 1-s window “i” and electrode “e” using Eq. (1).
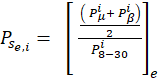
where Pse,i is the normalized power band on each electrode “e” on the 1-s-window “i” during mental state “s”. In this stage, “e” corresponds to C3 and C4. Pμi, Pβi and P8-30i are power bands in μ (8-12 Hz), β (18-24 Hz), and full range (8-30 Hz). A subtraction between Pse,c4and Pse,c3is calculated and an interpolation is considered to show this subtraction as NF on the screen with (2).
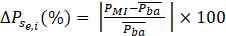
where ΔPse,i represents the normalization including MI trials as well as the mean P from the baseline period of all trials.
Once the patient has worn the EEG cap, he was instructed to imagine moving his paralyzed arm (left arm) for 2 min. To determine the frequency associated with his MI, the EEG data was processed in EEGLab/Matlab for mu and beta rhythms (from 8 to 30 Hz). EEG was evaluated in terms of energy decrease/increase in the rhythms mu and beta, which are related to MI.
2.3 tACS application
In each session, tACS is applied to the patient for 10 min and with current intensity of 0.4 mA. This application is done bilaterally, through conductive rubber electrodes (35 cm2), which are connected to perforated sponges soaked in saline solution connected to the tACS device (NeuroMyst Pro, USA).
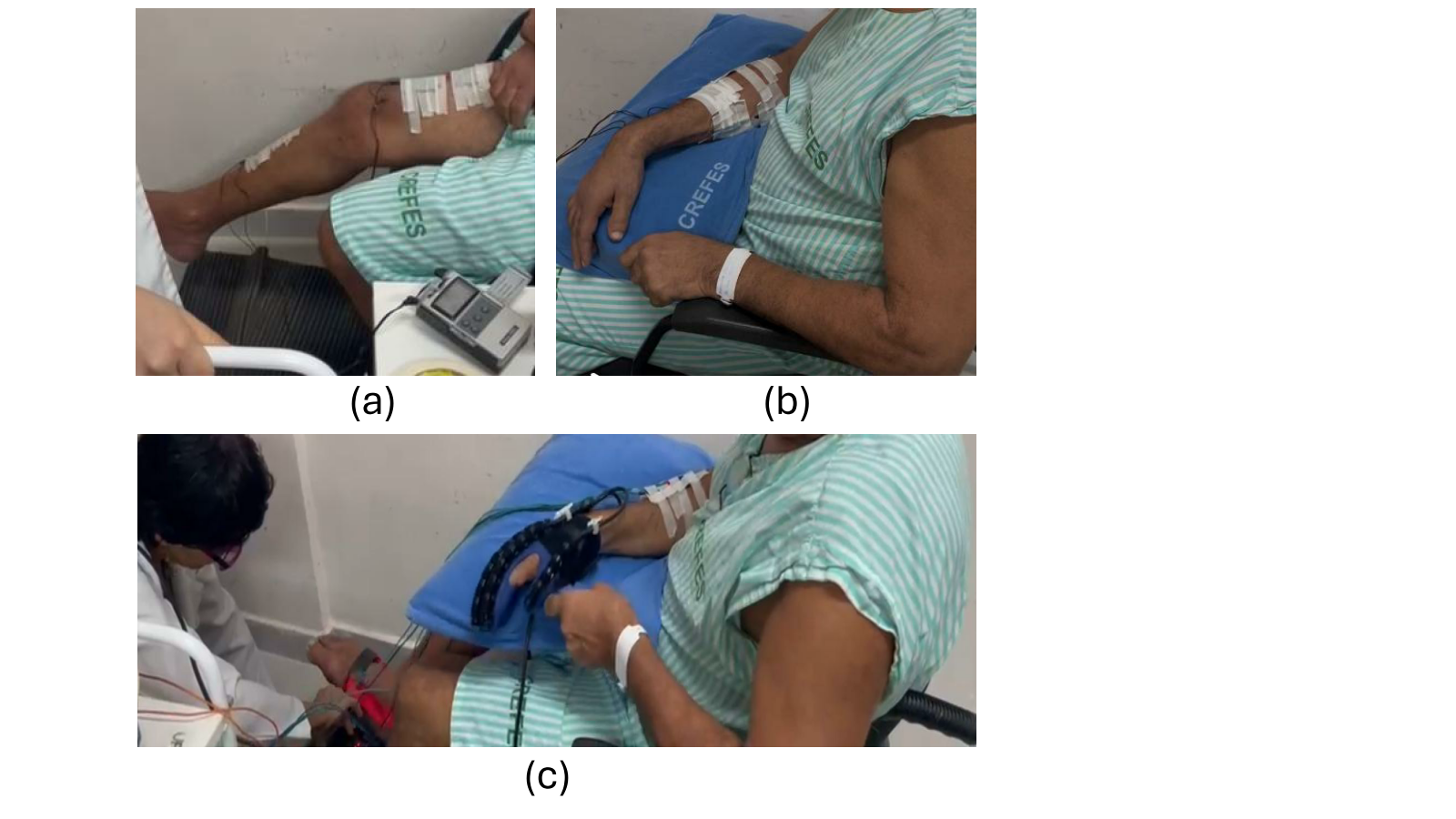
Figures 4(a) and 4(b) show the patient receiving tACS. It worth mentioning that during the tACS application the patient is instructed to conduct MI of the paralyzed limbs while watches a videoclip showing a person strolling in a field.
2.4 FES and robotic devices
Once the tACS procedure is completed, the patient is prepared for the next step of the protocol, which is to receive electrical stimulation from the FES device as well as passive movements from both the robotic monocycle and the robotic glove during MI. For the electrical stimulation, an FES device (Balego, USA) was used to target the finger flexors and extensors simultaneously, with the following parameters: alternating mode, 1-s ramp, 7-s on-time, 12-s off-time, frequency of 45 Hz, and pulse width of 250 µs. For the rectus femoris and tibialis anterior muscles, the following parameters were used: synchronous mode, 1-s ramp, 7-s on-time, 12-s off-time, frequency of 60 Hz, and pulse width of 300 µs. The stimulation intensity was adjusted based on the patient’s comfort level.
Fig 5 shows the patient with the FES electrodes attached to his paralyzed arm and leg, as well as his hand wearing the robotic glove and his feet on the robotic monocycle. Thus, whenever the patient performed MI, he received electrical stimulation and passive movements on the paralyzed limbs. The approach to recognize the patient’s MI was based on our previous work [22].
2. 5 Motor evaluation metrics
The patient’s rehabilitation progress was evaluated in terms of his motor ability, the metrics presented in Figure 1 and detailed in the following section were employed:
- Fugl-Meyer Assessment Scale (FMS)
Based on neurological examination and sensorimotor activity of upper and lower-limbs [23] evaluating: range of motion, pain, sensitivity, upper and lower extremity motor function, balance, coordination and speed. This scale has a total of 126 points for the upper-limb assessment, and 100 points for the lower-limb, totalling 226 points. To assess the percentage of recovery the following equation is used: % recovery = (Score Obtained x 100)/C; where C = 126 for upper limb and C = 100 for lower limb.
- Functional Independence Measurement (FIM)
Assesses a person's performance in the motor and cognitive/social domains feeding, personal hygiene, bathing, dressing the upper half of the body, dressing the lower half of the body, toilet use, urine control, fecal control, transfers to bed, chair, wheelchair, toilet transfers, bath or shower transfers, locomotion, stair locomotion, comprehension, expression, social interaction, problem solving, and memory [24]. Each of these items varies in seven levels with level 7 being total independence, and level 1 total dependence, modified independence (level 6), moderate dependence with the need for supervision or preparation (level 5) or with direct help (levels 1 to 4). A person without any disability achieves a score of 126 points, and one with total dependence scores 18 points. The more dependent, the lower the score.
- Modified Ashworth Scale (MAS)
To assess the intensity of hypertonia and therapeutic response the muscle resistance to passive movement is evaluated in degrees, as follows: Grade 0: classified as no increase in muscle tone; Grade 1: slight increase in muscle tone, manifested by grasping and releasing, or by minimal resistance at the end of the range of motion, when the affected limb (or limbs) is moved in flexion and extension; Grade 1+: slight increase in muscle tone, manifested by apprehension, followed by minimal resistance through the rest (less than half) of the range of motion; Grade 2: marked increase in muscle tone through most of the range of motion, but the affected limbs are easily moved; Grade 3: considerable increase in muscle tone, hindered passive movements; Grade 4: the affected limb (or limbs) are stiff on flexion or extension [24].
- MiniBESTest (MBT)
14 items that are grouped into four items: 1) Anticipatory postural adjustments; 2) Reactive postural responses; 3) Sensory orientation; 4) Gait stability with and without a cognitive task. Each item is scored on a three-point scale: from zero (worst performance) to two (best performance). The total score is 28 points, indicating that there is no deficit in dynamic balance. If the individual scores below 28 points, it means that there is a deficit. This test is useful for screening for deficits in dynamic balance and can be applied to individuals affected by various diseases and of any age [25].
- Modified Rankin Scale (mRS)
To assess the patient's level of disability globally, and, consequently, their level of functional dependence the levels of disability are classified as: 0: No symptoms; 1: No significant disability; 2: Mild disability; 3: Moderate disability; 4: Moderately severe disability; 5: Severe disability; 6: Death [26].
- Time Up and Go (TUG)
A functional mobility test that assesses gait, in function of postural and direction changes during the act of walking. The test consists of getting up from a chair with a backrest, without supporting the arms, walking 3 m, turning around, returning and sitting down again. TUG is used to assess the risk of falls. The time spent to perform this metrics is measured, which generates a risk classification, being low risk of falling (< 10 s), medium risk of falling (10-20 s), and high risk of falling (> 20 s) [27].
- 10-Meter Walk Test (10MWT)
An assessment of a person's mobility, speed, and ability to walk. This test consists of walking 10 m as fast as possible, without running. During the test, the time to cover the 10 m is timed, as well as the number of steps needed to complete the course [28].
- National Institutes of Health Stroke Scale (NIHSS)
An indicative of impairment level. This scale is composed of 11 items, each of which scores a specific ability between a 0 and 4 [29]. For each item, a score of 0 indicates normal function while a higher score is indicative of some level of impairment. The individual scores from each item are summed to calculate a patient's
- Barthel Index (BI)
An index of independence to accomplish ADL. The scale describes 10 tasks (feeding, bathing, grooming, dressing, bowel, bladder, toilet use, transfers bed-to-chair-and-back, mobility on level surfaces, and stair negotiation), which is scored according to amount of time or assistance required by the patient. Total score is from 0 to 100, with lower scores representing greater nursing dependency [30].
- surface electromyography (sEMG)
A technique that analyses the electrical activity of muscles. It is a non-invasive and painless method that can be used to monitor and assess muscle contraction [31].
- electroencephalograpy (EEG)
A technique that provides a direct measure of the functional neuroelectric activity in the brain, forming the basis for neuroplasticity and recovery of post-stroke patients, increasing prognostic ability [32].
3. Results
3.1 MI frequencies
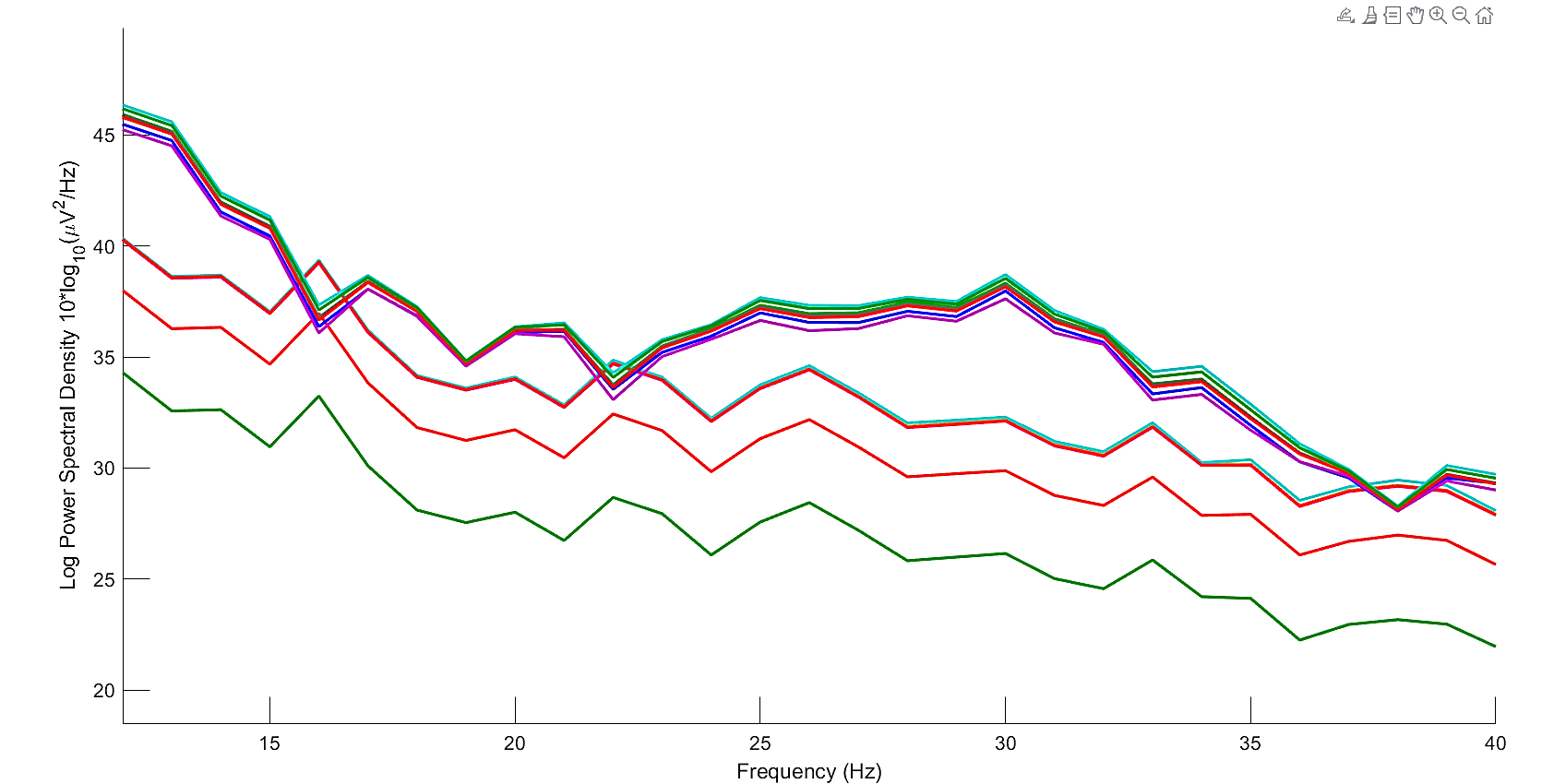
Peak values of Power Spectral Density (PSD) were found in the range of 8 to 30 Hz, as shown in Fig. 6. However, based on studies by [10], more efficiency of tACS is found for frequencies in beta band. Thus, we select the peak of 30 Hz as the stimulation frequency for the tACS device.
3.2 Motor evaluation results
Using FMS, the results indicate a gain of about 6% (Fig 7) in the recovery of the patient’s upper-limb function (functionality, range of motion, sensitivity, pain, proprioception and speed).
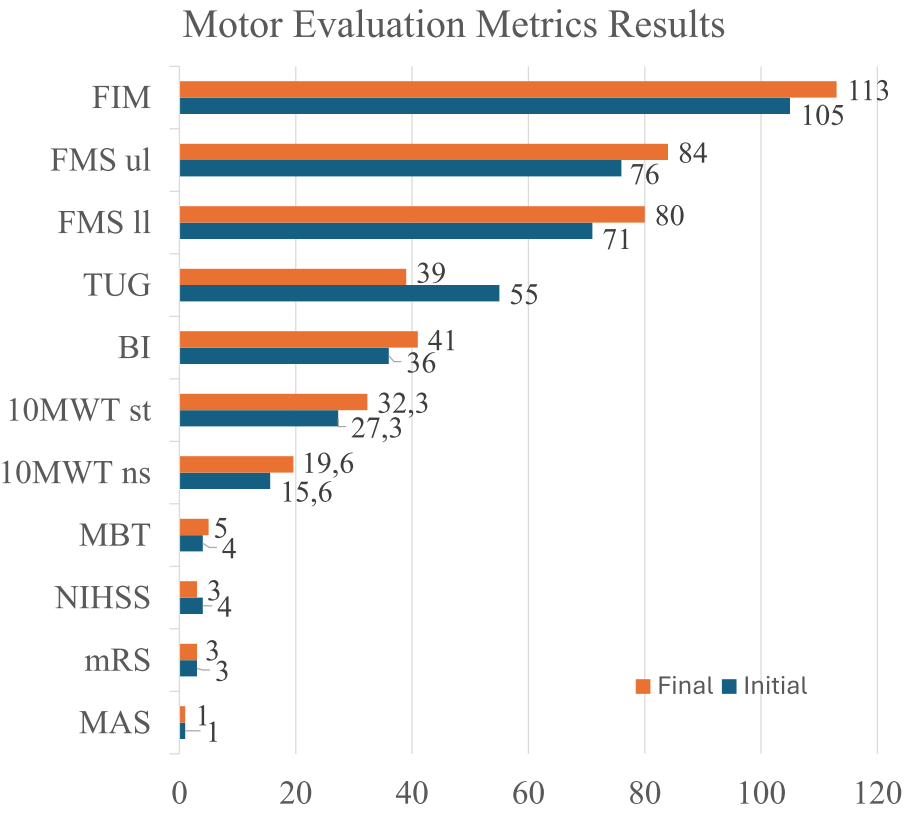
The FMS for lower-limb was also applied, with the patient improving 12.7% from the initial evaluation to the final evaluation. For FIM, the patient increased about 8% from the initial to the final evaluation. Also, in the initial evaluation the patient was evaluated with a score of 5.83 (moderate dependence with the need for supervision or preparation), whereas in the final evaluation, he improved his scored to 6.27, classifying him as a condition of “modified independence”. Related to MAS, the patient presented Grade 1 in both the initial and final evaluation. For MBT, two out of the four items were evaluated, which were item 1 and item 2, where the total score was 12 points. Thus, in the initial evaluation, the patient achieved a total score of 4 points (moderate balance deficit), whereas in the final evaluation, he achieved 5 points (moderate balance deficit), meaning 25% increase. For mRS, in both the initial evaluation and final evaluation the patient achieved a score of 3. Related to TUG metric, in the initial evaluation, the patient lasted 55 s to perform the TUG, whereas in the final evaluation he reached 39 s. It is worth mentioning that although in both evaluations the patient remained at high risk of falling (time >20 s), there was a significant reduction in the time to perform the test (reduction of 16 s, i.e., ≃ 30% in the time to perform the TUG when compared to the initial evaluation). For 10MWT, in the initial evaluation the patient lasted 27.33 s to walk 10 m (gait speed of 0.37 m/s), with approximately 16 steps. In the final evaluation, he lasted 32.33 s (gait speed of 0.31 m/s), with approximately 20 steps, resulting in 18% increase in time, and 25% increase in number of steps to walk 10 m. Fig. 6h-6i show these results. Related to NIHSS, in the neurological physical examination at the initial evaluation, the patient presented right hemiparesis, with grade 2 strength in his right hand, grade 3 in the rest of the right upper-limb, and grade 3 in his right lower-limb, scoring a 4 on NIHSS. In the final evaluation, he scored 3, representing 25% decrease. For BI, in the initial neurological physical examination the patient presented 36 points, whereas in the final evaluation he scored 41, representing 14% increase.
3.3 sEMG
Peak of fingers’ flexor muscle contraction, extensors, rectus femoris and tibialis anterior muscles were evaluated. An improvement in the muscle contraction of the finger flexors was observed, which had a peak of 78.35 μV in the initial evaluation, and 82.23 μV in the final evaluation (Fig. 8), representing ≃5% increase.
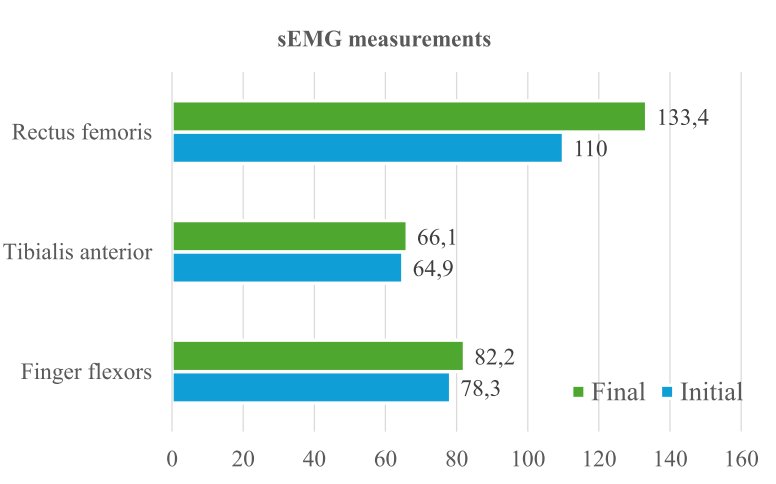
The tibialis anterior muscle presented a peak of 64.92 μV in the initial evaluation, and 66.06 μV in the final evaluation, representing ≃2% increase; and the rectus femoris muscle had a peak of 110.02 μV in the initial evaluation, and 133.43 μV in the final evaluation, representing ≃ 21%. However, the fingers’ extensor muscles did not show improvement in this evaluation.
3.4 EEG
Fig 9 illustrates the mean P values in electrodes C3 and C4 during Motor Imagery (MI) and Neurofeedback (NF) trials, analysed across mu, low-beta, and high-beta frequency bands.
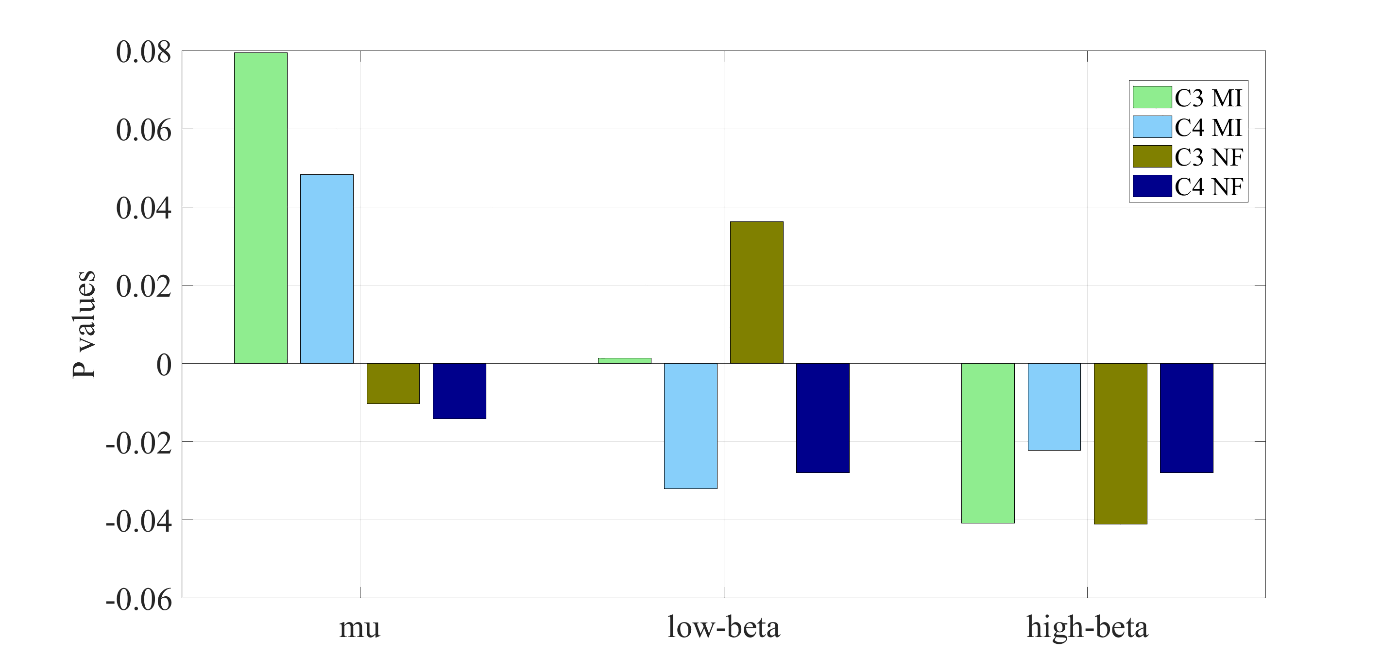
In the mu band, P values in C3 and C4 decreased during NF, reaching negative values, whereas they remained positive during MI without feedback. In the low-beta band, P values increased in C3 but became negative in C4 when comparing MI and NF. In contrast, high-beta exhibited a distinct pattern, with consistently negative P values in both C3 and C4 across MI and NF conditions, though lower P values were observed during NF. Negative P values suggest stronger engagement or Event-Related Desynchronization (ERD), which is often desirable in MI-based BCIs, reinforcing the idea that NF enhances MI performance compared to MI alone. Regarding the energy variation in mu and beta bands,
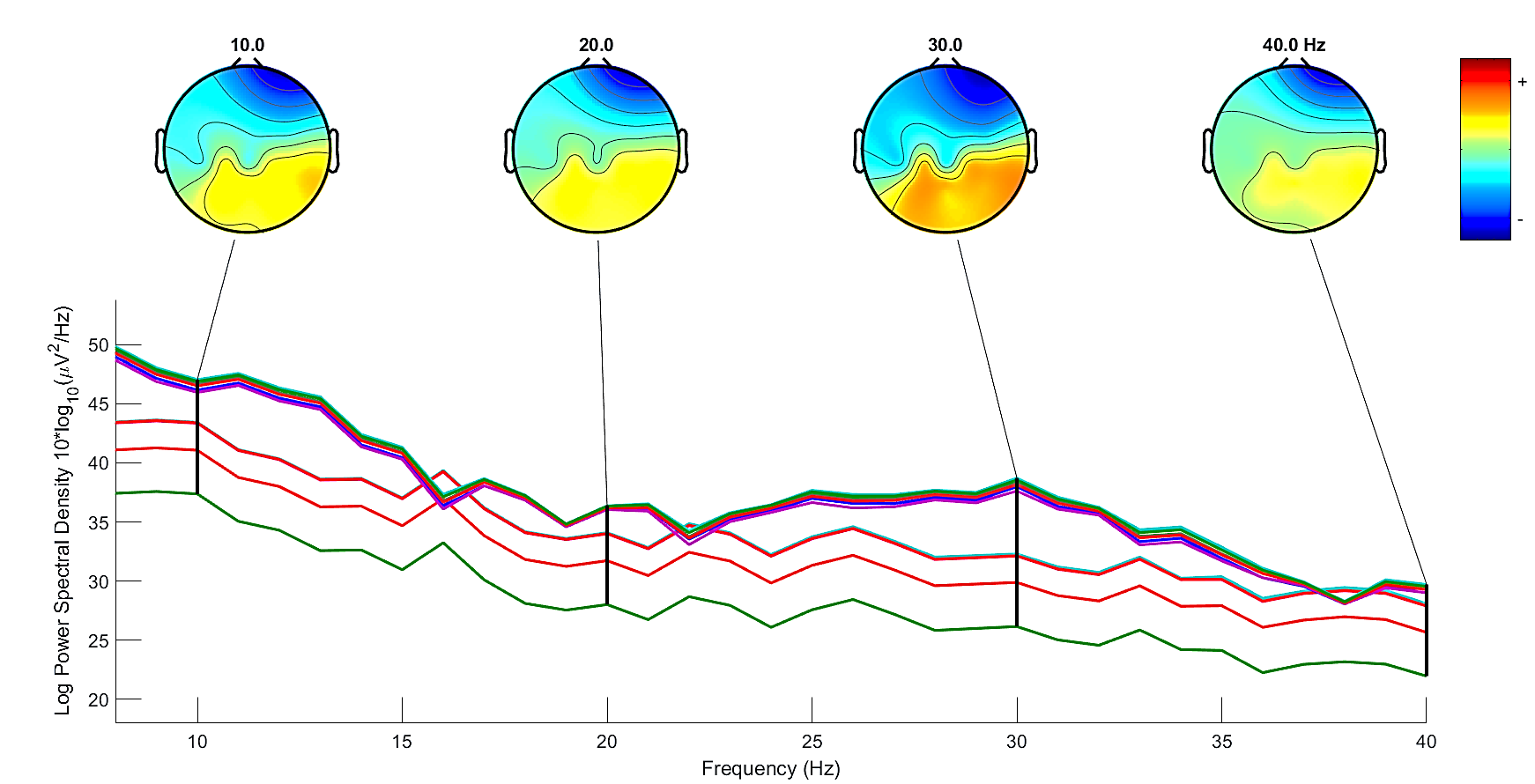
Fig 10 shows the topographic maps captured before and after the intervention, in which it is possible to see the increase of energy (red colour) at the end of intervention.
4. Discussion and Conclusions
Results of this study demonstrate that the patient improved his condition at the end of the intervention (except for 10MWT), where he had 18% increase in time and 25% increase in number of steps. However, in all additional metrics he improved his condition. For instance, in Fugl-Meyer Assessment Scale (FMS) he had both 6% increase for upper-limb and 9% increase for lower-limb; in Functional Independence Measurement (FIM) he improved 8%, and passing from a FIM score of 5.83 to 6.27 in MiniBESTest (MBT) he had 25% increase; in Time Up and Go (TUG) he had 30% decrease; in National Institutes of Health Stroke Scale (NIHSS) he had 25% decrease; and in Barthel Index (BI) he had 14% increase. Nevertheless, there was not variation (comparing the initial and the final evaluation) in Modified Ashworth Scale (MAS) (which maintained the Grade 1) and modified Rankin Scale (mRS) (which maintained a score of 3). Regarding the results for surface Electromyography (sEMG), there was 5% increase in muscle contraction peak for finger flexors, 2% for tibialis anterior and 21% for rectus femoris.
For the EEG analysis, topographic maps showed increase of energy ratio in beta/mu rhythms at the end of intervention, whose results also indicate that the use of NF enhanced MI performance compared to MI alone.
From these results, we do believe that these improvements were due to our therapy, which facilitated neuromodulation, enhancing the excitability of the motor cortex and improving muscle activation, as well as reducing spasticity and allowing for greater voluntary contraction during evaluations.
The reduction in gait speed observed in the 10-Meter Walk Test (10MWT) may be due to a typical adaptation in the locomotor pattern of stroke patients, in which they begin to prioritize postural control and gait stability over gross speed. This hypothesis is supported by the fact that all other functional tests of our patient showed improvement. In the Timed Up and Go (TUG) results, for example, a reduction in the patient's execution time was observed, indicating an improvement in functional capacity related to mobility. Furthermore, the results of the FMS for the lower limbs and the MBT indicate gains in muscle strength and balance, respectively, which reinforces the hypothesis of improved postural control and stability during activities of daily living, including gait.
The robotic technology was used for both upper and lower limb motor rehabilitation, demonstrating good reliability in terms of ease of use, anthropometric adaptation, and robustness, in line with the growing trend of employing robot-assisted devices such as orthoses and pedalling systems, as well as brain and muscle electrical stimulation, which provide valuable feedback in post-stroke rehabilitation as reported in the literature [9], [10], [11], [12], [13].
In this study, functional assessment was carried out using clinical scales complemented by sEMG and EEG techniques to provide objective measurements, underscoring the importance of integrating multimodal recording systems in post-stroke evaluation [24], [28]. By implementing the rehabilitation technology in a real clinical setting, this work addresses one of the major challenges identified in the field, namely the translation of advanced neurorehabilitation approaches into routine clinical practice.
The technology employed has enabled the design of a personalized protocol with a combined approach, integrating rehabilitation tasks that include motor activity through low-complexity exercises, providing movement assistance, stimulating neuroplasticity, using visual feedback, as addressed in other studies [9]. It is important to establish in the protocol an adequate number of repetitions for each patient to promote motor learning without inducing fatigue and to work with robust BCI systems with low sensitivity to noise, as recommended in preliminary studies [12], [13]. In light of these scenarios, it is essential to continue advancing AI models as integral components of practical protocols that support clinical decision-making and patient follow-up [11].
It is worth commenting that there were limitations in this study, which evaluated only a single participant without control group, sham protocol and blinding therapy. This study also did not present comparisons with other studies, as we believe that this is the first study using a combination of tACS, MI-BCI-NF, FES and robotic devices for post-stroke rehabilitation. Therefore, more studies are needed to confirm the results with a wider sample, control group, sham therapy, as well as conducting the research by blinded evaluators.
Acknowledgements
The authors thank the Rehabilitation Center of Espirito Santo (CREFES) for making available the patients for this research, and FAPES (Project Number: 983/2022 P: 2022-B92KF) and CNPq (all from Brazil) for funding this research.
References
[1] U. N. D. of Economic y S. Affairs, World population ageing 2020: highlights: living arrangements of older persons. UN, 2021.
[2] CDC, «Risk Factors for Stroke», Stroke. Accessed: 20 de agosto de 2025. [Online]. Available: View Article
[3] CDC, «About Stroke», Stroke. Accessed: 20 de agosto de 2025. [Online]. Available: View Article
[4] V. F. Cardoso et al., «Effect of a Brain-Computer Interface Based on Pedaling Motor Imagery on Cortical Excitability and Connectivity», Sensors, vol. 21, n.o 6, p. 2020, ene. 2021, doi: 10.3390/s21062020. View Article
[5] T. Bastos-Filho, Introduction to Non-Invasive EEG-Based Brain-Computer Interfaces for Assistive Technologies. CRC Press, 2020. doi: 10.1201/9781003049159. View Article
[6] W. Yan, Y. Lin, Y.-F. Chen, Y. Wang, J. Wang, y M. Zhang, «Enhancing Neuroplasticity for Post-Stroke Motor Recovery: Mechanisms, Models, and Neurotechnology», IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 33, pp. 1156-1168, 2025, doi: 10.1109/TNSRE.2025.3551753. View Article
[7] N. Aderinto, M. O. AbdulBasit, G. Olatunji, y T. Adejumo, «Exploring the transformative influence of neuroplasticity on stroke rehabilitation: a narrative review of current evidence», Annals of Medicine and Surgery, vol. 85, n.o 9, p. 4425, sep. 2023, doi: 10.1097/MS9.0000000000001137. View Article
[8] X. Li, Y. He, D. Wang, y M. J. Rezaei, «Stroke rehabilitation: from diagnosis to therapy», Front. Neurol., vol. 15, ago. 2024, doi: 10.3389/fneur.2024.1402729. View Article
[9] H. E. Shin et al., «Therapeutic Effects of Functional Electrical Stimulation on Physical Performance and Muscle Strength in Post-stroke Older Adults: A Review», Ann Geriatr Med Res, vol. 26, n.o 1, pp. 16-24, mar. 2022, doi: 10.4235/agmr.22.0006. View Article
[10] S. Yang, Y. G. Yi, y M. C. Chang, «The effect of transcranial alternating current stimulation on functional recovery in patients with stroke: a narrative review», Front. Neurol., vol. 14, ene. 2024, doi: 10.3389/fneur.2023.1327383. View Article
[11] S. R. Kopalli et al., «Artificial intelligence in stroke rehabilitation: From acute care to long-term recovery», Neuroscience, vol. 572, pp. 214-231, abr. 2025, doi: 10.1016/j.neuroscience.2025.03.017. View Article
[12] «Effects of task complexity or rate of motor imagery on motor learning in healthy young adults - Heena - 2021 - Brain and Behavior - Wiley Online Library». Accessed: 22 de agosto de 2025. [Online]. Available: https://onlinelibrary.wiley.com/doi/full/10.1002/brb3.2122 View Article
[13] M. S. Kim et al., «Efficacy of brain-computer interface training with motor imagery-contingent feedback in improving upper limb function and neuroplasticity among persons with chronic stroke: a double-blinded, parallel-group, randomized controlled trial», J NeuroEngineering Rehabil, vol. 22, n.o 1, p. 1, ene. 2025, doi: 10.1186/s12984-024-01535-2. View Article
[14] G. Pfurtscheller y F. H. Lopes da Silva, «Event-related EEG/MEG synchronization and desynchronization: basic principles», Clinical Neurophysiology, vol. 110, n.o 11, pp. 1842-1857, nov. 1999, doi: 10.1016/S1388-2457(99)00141-8. View Article
[15] B. C. M. van Wijk, «An Introduction to EEG/MEG for Model-Based Cognitive Neuroscience», en An Introduction to Model-Based Cognitive Neuroscience, B. U. Forstmann y B. M. Turner, Eds., Cham: Springer International Publishing, 2024, pp. 185-209. doi: 10.1007/978-3-031-45271-0_8. View Article
[16] «Effect of Neurofeedback Facilitation on Poststroke Gait and Balance Recovery | Neurology». Accessed: 22 de agosto de 2025. [Online]. Available: https://www.neurology.org/doi/full/10.1212/WNL.0000000000011989 View Article
[17] H. P. Adams et al., «Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment.», Stroke, vol. 24, n.o 1, pp. 35-41, ene. 1993, doi: 10.1161/01.STR.24.1.35. View Article
[18] J. Su et al., «An Adaptive Hybrid Brain-Computer Interface for Hand Function Rehabilitation of Stroke Patients», IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 32, pp. 2950-2960, 2024, doi: 10.1109/TNSRE.2024.3431025. View Article
[19] C. F. Blanco-Diaz, E. R. da S. Serafini, T. Bastos-Filho, A. F. O. de A. Dantas, C. C. do E. Santo, y D. Delisle-Rodriguez, «A Gait Imagery-Based Brain-Computer Interface With Visual Feedback for Spinal Cord Injury Rehabilitation on Lokomat», IEEE Transactions on Biomedical Engineering, vol. 72, n.o 1, pp. 102-111, ene. 2025, doi: 10.1109/TBME.2024.3440036. View Article
[20] A. X. G. Cely, C. F. Blanco-Diaz, C. D. G. Mendez, A. C. V. Parra, y T. F. Bastos-Filho, «Classification of opening/closing hand motor imagery induced by left and right robotic gloves through EEG signals», Transactions on Energy Systems and Engineering Applications, vol. 5, n.o 2, pp. 1-9, dic. 2024, doi: 10.32397/tesea.vol5.n2.579. View Article
[21] R. Zhang et al., «An Adaptive Brain-Computer Interface to Enhance Motor Recovery After Stroke», IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 31, pp. 2268-2278, 2023, doi: 10.1109/TNSRE.2023.3272372. View Article
[22] T. F. Bastos-Filho et al., «A novel methodology based on static visual stimuli and kinesthetic motor imagery for upper limb neurorehabilitation», Res. Biomed. Eng., vol. 40, n.o 3, pp. 687-700, oct. 2024, doi: 10.1007/s42600-024-00372-5. View Article
[23] D. J. Gladstone, C. J. Danells, y S. E. Black, «The Fugl-Meyer Assessment of Motor Recovery after Stroke: A Critical Review of Its Measurement Properties», Neurorehabil Neural Repair, vol. 16, n.o 3, pp. 232-240, sep. 2002, doi: 10.1177/154596802401105171. View Article
[24] T. Vidmar, N. Goljar Kregar, y U. Puh, «Reliability of the Modified Ashworth Scale After Stroke for 13 Muscle Groups», Archives of Physical Medicine and Rehabilitation, vol. 104, n.o 10, pp. 1606-1611, oct. 2023, doi: 10.1016/j.apmr.2023.04.008. View Article
[25] A.-B. Meseguer-Henarejos, J.-A. López-Pina, J.-J. López-García, y I. Martínez-González-Moro, «Psychometric properties of the Mini-Balance Evaluation Systems Test (Mini-BESTest) among multiple populations: a COSMIN systematic review and meta-analysis», Disability and Rehabilitation, vol. 0, n.o 0, pp. 1-24, doi: 10.1080/09638288.2025.2456602. View Article
[26] «Standardized Nomenclature for Modified Rankin Scale Global Disability Outcomes: Consensus Recommendations From Stroke Therapy Academic Industry Roundtable XI | Stroke». Accessed: Aug. 25, 2025. [Online]. Available: View Article
[27] «Timed Up & Go as a measure for longitudinal change in mobility after stroke - Postural Stroke Study in Gothenburg (POSTGOT) | Journal of NeuroEngineering and Rehabilitation». Accessed: Aug. 25, 2025. [Online]. Available: https://link.springer.com/article/10.1186/1743-0003-11-83 View Article
[28] D. K. Cheng, M. Nelson, D. Brooks, y N. M. Salbach, «Validation of stroke-specific protocols for the 10-meter walk test and 6-minute walk test conducted using 15-meter and 30-meter walkways», Topics in Stroke Rehabilitation, vol. 27, n.o 4, pp. 251-261, may 2020, doi: 10.1080/10749357.2019.1691815. View Article
[29] S. A. Kazaryan et al., «The National Institutes of Health Stroke Scale is comparable to the ICH score in predicting outcomes in spontaneous acute intracerebral hemorrhage», Front. Neurol., vol. 15, jul. 2024, doi: 10.3389/fneur.2024.1401793. View Article
[30] «The Validation Study of Both the Modified Barthel and Barthel Index, and Their Comparison Based on Rasch Analysis in the Hospitalized Acute Stroke Elderly - Reyhaneh Aminalroaya, Fatemeh Sadat Mirzadeh, Kazem Heidari, Mahtab Alizadeh-Khoei, Farshad Sharifi, Mohammad Effatpanah, Leila Angooti-Oshnari, Sadeqh Fadaee, Homan Saghebi, Sakar Hormozi, 2021». Accessed: Aug. 25, 2025. [Online]. Available: View Article
[31] K. M. Steele, C. Papazian, y H. A. Feldner, «Muscle Activity After Stroke: Perspectives on Deploying Surface Electromyography in Acute Care», Front. Neurol., vol. 11, sep. 2020, doi: 10.3389/fneur.2020.576757. View Article
[32] «The Prognostic Utility of Electroencephalography in Stroke Recovery: A Systematic Review and Meta-Analysis - Amanda A Vatinno, Annie Simpson, Viswanathan Ramakrishnan, Heather S. Bonilha, Leonardo Bonilha, Na Jin Seo, 2022». Accessed: Aug. 25, 2025. [Online]. Available: View Article

